Compound (10)
Article Title (1)
Article Author (12)
Assay (6)
Zhou, Y; Vu, K; Chen, Y; Pham, J; Brady, T; Liu, G; Chen, J; Nam, J; Murali Mohan Reddy, PS; Au, Q; Yoon, IS; Tremblay, MH; Yip, G; Cher, C; Zhang, B; Barber, JR; Ng, SC Bioorg Med Chem Lett 19: 3128 -35 (2009) Au, V; Harichian, B; Marrero, D US Patent US11202743 (2021) Toure, M; Johnson, T; Li, B; Schmidt, R; Ma, H; Neagu, C; Lopez, AU; Wang, Y; Guler, S; Xiao, Y; Henkes, R; Ho, K; Zhang, S; Chu, CL; Gundra, UM; Porichis, F; Li, L; Maurer, CK; Fang, Z; Musil, D; DiPoto, M; Friis, E; Jones, R; Jones, C; Cummings, J; Chekler, E; Tanzer, EM; Huck, B; Sherer, B Bioorg Med Chem 92: (2023) Chowdhury, SR; Kennedy, S; Zhu, K; Mishra, R; Chuong, P; Nguyen, AU; Kathman, SG; Statsyuk, AV Bioorg Med Chem Lett 29: 36 -39 (2019) Barlind, JG; Bauer, UA; Birch, AM; Birtles, S; Buckett, LK; Butlin, RJ; Davies, RD; Eriksson, JW; Hammond, CD; Hovland, R; Johannesson, P; Johansson, MJ; Kemmitt, PD; Lindmark, BT; Morentin Gutierrez, P; Noeske, TA; Nordin, A; O'Donnell, CJ; Petersson, AU; Redzic, A; Turnbull, AV; Vinblad, J J Med Chem 55: 10610 -29 (2012) Sultan, S; Choudhary, MI; Khan, SN; Fatima, U; Atif, M; Ali, RA; Rahman, AU; Fatmi, MQ Eur J Med Chem 62: 764 -70 (2013) Rao, AU; Shao, N; Aslanian, RG; Chan, TY; Degrado, SJ; Wang, L; McKittrick, B; Senior, M; West, RE; Williams, SM; Wu, RL; Hwa, J; Patel, B; Zheng, S; Sondey, C; Palani, A ACS Med Chem Lett 3: 198 -202 (2012) Zawawi, NK; Taha, M; Ahmat, N; Ismail, NH; Wadood, A; Rahim, F; Rehman, AU Bioorg Chem 63: 36 -44 (2015) White, BH; Whalen, K; Kriksciukaite, K; Alargova, R; Au Yeung, T; Bazinet, P; Brockman, A; DuPont, M; Oller, H; Lemelin, CA; Lim Soo, P; Moreau, B; Perino, S; Quinn, JM; Sharma, G; Shinde, R; Sweryda-Krawiec, B; Wooster, R; Bilodeau, MT J Med Chem 62: 2708 -2719 (2019) IJzerman, AP; Aué, GH; Bultsma, T; Linschoten, MR; Timmerman, H J Med Chem 28: 1328 -34 (1985) Nasib, A; Musharraf, SG; Hussain, S; Khan, S; Anjum, S; Ali, S; Atta-Ur-Rahman, AU; Choudhary, MI J Nat Prod 69: 957 -9 (2006) Féau, C; Arnold, LA; Kosinski, A; Zhu, F; Connelly, M; Guy, RK ACS Chem Biol 4: 834 -43 (2009)
ChEMBL_1707813 (CHEMBL4059046) Binding affinity to human full length recombinant His-tagged HuR expressed in Escherichia coli Rosetta DH5alpha assessed as inhibition of interaction with single-strand Bi-AU RNA probe by AlphaScreen assay Human DHODH Inhibition Assay The in vitro inhibition of hDHODH was measured using an N-terminally truncated recombinant hDHODH enzyme as described in J. Med. Chem. 2006; 49:1239. Briefly, the hDHODH concentration was adjusted in a way that an average slope of approximately 0.2 AU/min served as the positive control (e.g. without inhibitor). The standard assay mixture contained 60 μM 2,6-dichloroindophenol, 50 μM decylubiquinone and 100 μM dihydroorotate. The hDHODH enzyme with or without at least six different concentrations of the compounds was added and measurements were performed in 50 mM TrisHCl, 150 mM KCl and 0.1% Triton X-100 at pH 8.0 and at 30° C. The reaction was started by adding dihydroorotate and measuring the absorption at 600 nm for 2 min. Human DHODH Inhibition Assay The in vitro inhibition of hDHODH was measured using an N-terminally truncated recombinant hDHODH enzyme as described in J. Med. Chem. 2006; 49:1239. Briefly, the hDHODH concentration was adjusted in a way that an average slope of approximately 0.2 AU/min served as the positive control (e.g. without inhibitor). The standard assay mixture contained 60 μM 2,6-dichloroindophenol, 50 μM decylubiquinone and 100 μM dihydroorotate. The hDHODH enzyme with or without at least six different concentrations of the compounds was added and measurements were performed in 50 mM TrisHCl, 150 mM KCl and 0.1% Triton X-100 at pH 8.0 and at 30° C. The reaction was started by adding dihydroorotate and measuring the absorption at 600 nm for 2 min. For the determination of the IC50 values, each data point was recorded in triplicate. For the determination of the inhibitory constant Ki, the KM values for DHO and decylubichinon were determined. Afterwards, the compounds were diluted in a dilution series depending on their IC50 values in DMSO. Human DHODH Inhibition Assay The in vitro inhibition of hDHODH was measured using an N-terminally truncated recombinant hDHODH enzyme as described in J. Med. Chem. 2006; 49:1239. Briefly, the hDHODH concentration was adjusted in a way that an average slope of approximately 0.2 AU/min served as the positive control (e.g. without inhibitor). The standard assay mixture contained 60 UM 2,6-dichloroindophenol, 50 μM decylubiquinone and 100 UM dihydroorotate. The hDHODH enzyme with or without at least six different concentrations of the compounds was added and measurements were performed in 50 mM TrisHCI, 150 mM KCl and 0.1% Triton X-100 at pH 8.0 and at 30° C. The reaction was started by adding dihydroorotate and measuring the absorption at 600 nm for 2 min. For the determination of the IC50 values, each data point was recorded in triplicate. For the determination of the inhibitory constant Ki, the KM values for DHO and decylubichinon were determined. Afterwards, the compounds were diluted in a dilution series depending on their IC50 values in DMSO. KHK-C Assay A A 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepared in DMSO as 4 mM stocks, diluted using an 11-point half-log scheme on a Biomek FX (Beckman Coulter), and incubated at rt for 30 minutes with the reaction mixture containing 50 mM HEPES, pH 7.4, 140 mM KCl, 3.5 mM MgCl2, 0.8 mM fructose, 2 mM TCEP, 0.8 mM PEP, 0.7 mM NADH, 0.01% Triton X-100, 30 U/mL pyruvate kinase-lactate dehydrogenase, and 10 nM purified KHK-C. The compound concentration in each well ranged from 1 nM to 100 μM. The reaction was initiated with the addition of 0.2 mM ATP. The absorbance was measured for 30 minutes on a SpectraMax reader (Molecular Devices) after ATP was added. The concentrations provided are based on the final mixture volume of 40 μL (referred to as the final concentration). Controls: N8-(cyclopropylmethyl)-N4-(2-(methylthio)phenyl)-2-(piperazin-1-yl)pyrimido[5,4-d]pyrimidine-4,8-diamine at 2 μM final concentration was used as high percent effect (HPE) control, and 2.5% DMSO which was present in all reaction wells was used as zero percent effect (ZPE) control. Reaction rates were obtained for 300-1800 seconds time window in units of 1000*AU/min (absorbance unit per minute), and average values for ZPE and HPE controls from 16 wells each were calculated, AveZPE and AveHPE, respectively. KHK Assay A A 384-well format on a Corning 3653 assay plate is used, and monitored by UV-vis spectroscopy in continuous mode at rt. Compounds were prepared in DMSO as 4 mM stocks, diluted using an 11-point half-log scheme on a Biomek FX (Beckman Coulter), and incubated at rt for 30 minutes with the reaction mixture containing 50 mM HEPES, pH 7.4, 140 mM KCl, 3.5 mM MgCl2, 0.8 mM fructose, 2 mM TCEP, 0.8 mM PEP, 0.7 mM NADH, 0.01% Triton X-100, 30 U/mL pyruvate kinase-lactate dehydrogenase, and 10 nM purified KHK-C. The compound concentration in each well ranged from 1 nM to 100 μM. The reaction was initiated with the addition of 0.2 mM ATP. The absorbance was measured for 30 minutes on a SpectraMax reader (Molecular Devices) after ATP was added. The concentrations provided are based on the final mixture volume of 40 μL (referred to as the final concentration).Controls: N8-(cyclopropylmethyl)-N4-(2-(methylthio)phenyl)-2-(piperazin-1-yl)pyrimido[5,4-d]pyrimidine-4,8-diamine at 2 μM final concentration was used as high percent effect (HPE) control, and 2.5% DMSO which was present in all reaction wells was used as zero percent effect (ZPE) control. Reaction rates were obtained for 300-1800 seconds time window in units of 1000*AU/min (absorbance unit per minute), and average values for ZPE and HPE controls from 16 wells each were calculated, AveZPE and AveHPE, respectively.Percent inhibition (% inhibition) was calculated for each well using this equation:100 - 100 × ( Compound absorbance rate value - Ave HPE ) ( Ave ZPE - Ave HPE )The % inhibition was then plotted against the log of compound concentration using GraphPad Prism, and the data was fit to the equation log [compound] vs. response variable slope using nonlinear regression analysis to give IC50 values. For each compound tested, the IC50 provided is the average based on at least two separate assays conducted on separate days.
 BDBM204987 US9249085, I(au)
BDBM204987 US9249085, I(au)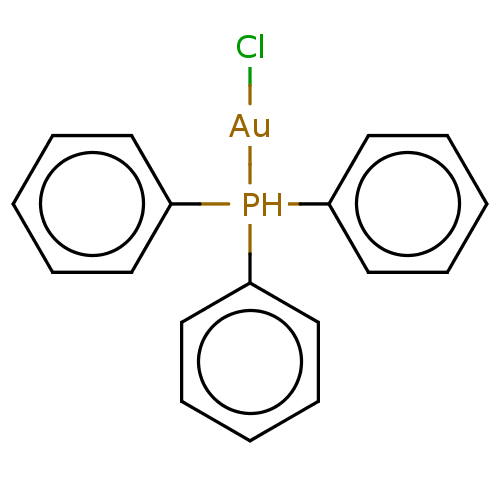 BDBM234409 [Au(tpp)Cl] (1)
BDBM234409 [Au(tpp)Cl] (1) BDBM660467 US20240092799, Compound XXIX-AU
BDBM660467 US20240092799, Compound XXIX-AU US12325697, Compound AU-2 BDBM747530
US12325697, Compound AU-2 BDBM747530 [Au(CN)2]- CHEMBL180870 BDBM50164086
[Au(CN)2]- CHEMBL180870 BDBM50164086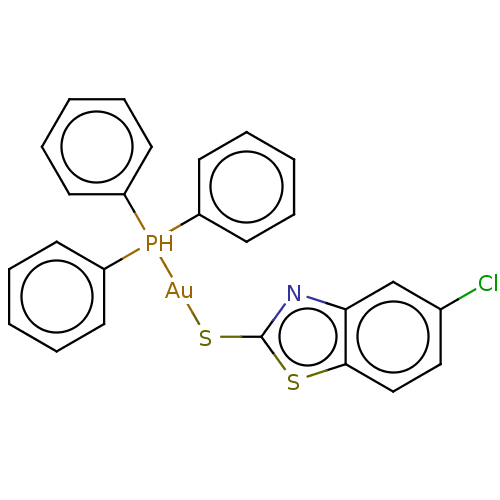 [Au(tpp)(Clmbzt)] (4) BDBM234412
[Au(tpp)(Clmbzt)] (4) BDBM234412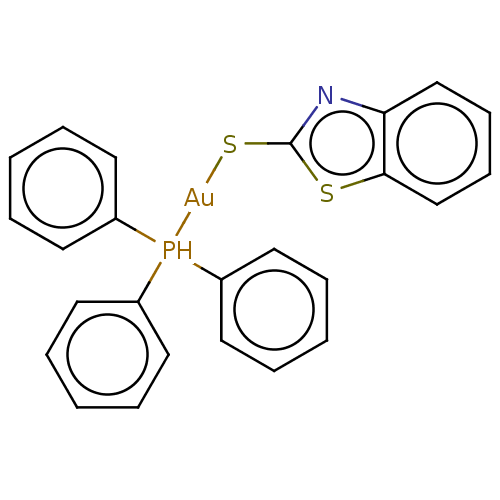 [Au(tpp)(mbzt)] (3) BDBM234411
[Au(tpp)(mbzt)] (3) BDBM234411 [Au(tpp)(mtzd)] (2) BDBM234410
[Au(tpp)(mtzd)] (2) BDBM234410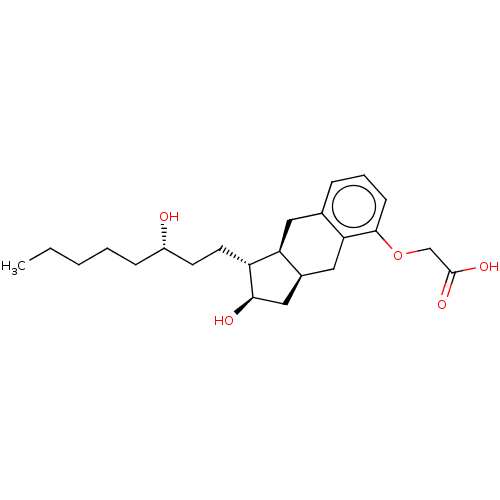 15-AU-81 Remodulin LRX-15 Uniprost TREPROSTINIL CHEBI:50861 UT-15 Tresprostinil Tyvaso dpi BDBM50594971 LRX -15 15AU81 Rumodolin Tyvaso L-606
15-AU-81 Remodulin LRX-15 Uniprost TREPROSTINIL CHEBI:50861 UT-15 Tresprostinil Tyvaso dpi BDBM50594971 LRX -15 15AU81 Rumodolin Tyvaso L-606 2-(2,6-dichloro-3-((3-oxomorpholino)methyl)benzyl)-1,4-dimethyl-1H-indole-6-carbonitrile Example AU1: 2-(2,6-dichloro-3-((3-oxomorpholino)methyl)benzoyl)-1,4-dimethyl-1H-indole-6-carbonitrile BDBM293088 US10106501, Example AU
2-(2,6-dichloro-3-((3-oxomorpholino)methyl)benzyl)-1,4-dimethyl-1H-indole-6-carbonitrile Example AU1: 2-(2,6-dichloro-3-((3-oxomorpholino)methyl)benzoyl)-1,4-dimethyl-1H-indole-6-carbonitrile BDBM293088 US10106501, Example AU