 Apatinib BDBM50152828 YN-968D1
Apatinib BDBM50152828 YN-968D1 APS-3-77 US10548897, Compound 50 BDBM429415
APS-3-77 US10548897, Compound 50 BDBM429415 US10548897, Compound 10 APS-2-12 BDBM429418
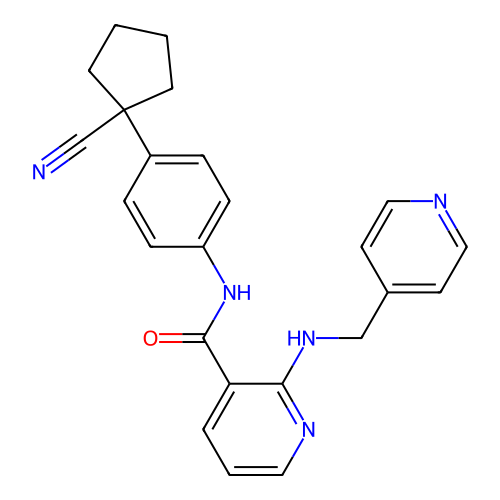
US10548897, Compound 10 APS-2-12 BDBM429418 US10548897, Compound 21 BDBM429286 APS-2-79
US10548897, Compound 21 BDBM429286 APS-2-79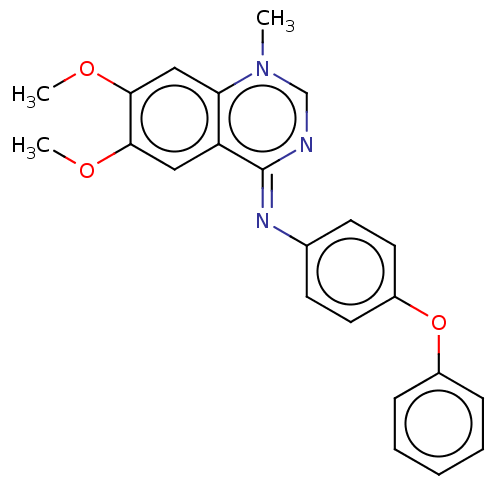 US10548897, Compound 32 BDBM429421 APS-3-6
US10548897, Compound 32 BDBM429421 APS-3-6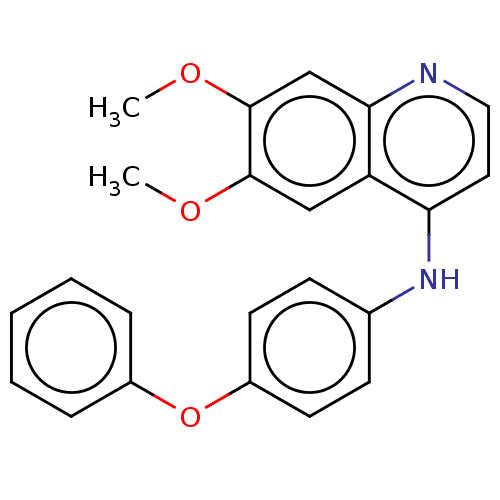 US10548897, Compound 11 BDBM429420 D3RKN_87 APS-2-16
US10548897, Compound 11 BDBM429420 D3RKN_87 APS-2-16 Adenosine Phosphosulfate (APS) BDBM25461 Adenosine 5-Phosphosulfate Adenylyl sulfate [({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]sulfonic acid
Adenosine Phosphosulfate (APS) BDBM25461 Adenosine 5-Phosphosulfate Adenylyl sulfate [({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]sulfonic acid
- ChEMBL_303625 (CHEMBL828836) Inhibition of [3H]APS-314d binding to prostacyclin receptors (IP) of human platelet membrane
- Luminescent assay for HTS discovery of chemical activators of placental alkaline phosphatase Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screening Centers Network (MLSCN) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified: three isozymes are tissue-specific and the fourth one is tissue-nonspecific. Placental alkaline phosphatase (PLAP) is highly expressed in primate placental tissue. Its biological function is unknown. Identification of PLAP-specific inhibitors should provide necessary tools for characterization of its biological role. PLAP screening was developed and performed at the Sanford-Burnham Center for Chemical Genomics (SBCCG) as part of the Molecular Library Screening Center Network (MLSCN). This assay represents a selectivity screening for tissue nonspecific alkaline phosphatase (TNAP) screened at BCC
- Luminescent assay for HTS discovery of chemical inhibitors of placental alkaline phosphatase Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screening Centers Network (MLSCN) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified: three isozymes are tissue-specific and the fourth one is tissue-nonspecific. Placental alkaline phosphatase (PLAP) is highly expressed in primate placental tissue. Its biological function is unknown. Identification of PLAP-specific inhibitors should provide necessary tools for characterization of its biological role. PLAP screening was developed and performed at the Sanford-Burnham Center for Chemical Genomics (SBCCG) as part of the Molecular Library Screening Center Network (MLSCN). This assay represents a selectivity screening for tissue nonspecific alkaline phosphatase (TNAP) screened at B
- SAR analysis of an In Vitro TNAP Dose Response Luminescent Assay Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Number: MH077602-01 Assay Provider: Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute, San Diego, CA This TNAP dose response assay is developed and performed for the purpose of SAR study on analogs of hits originally identified in the TNAP luminescent HTS assay (AID 518) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in the most organism. In human, four isozymes of APs have been identified. Three isozymes are tissue-specific and the fourth one is tissue-nonsepecifc, named TNAP. TNAP deficiency is associated with defective bone mineralization in the form of rickets and osteomalacia. Therefore, there are therapeutic potentials of inhibiting TNAP activity
- TNAP luminescent HTS assay Sanford-Burnham Center for Chemical Genomics (SBCCG) Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Number: MH077602-01 Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in the most organism. In human, four isozymes of APs have been identified. Three isozymes are tissue-specific and the fourth one is tissue-nonsepecifc, named TNAP. TNAP deficiency is associated with defective bone mineralization in the form of rickets and osteomalacia. Therefore, there are therapeutic potentials of inhibiting TNAP activity. The goal of this HTS is to identify novel and specific inhibitors of TNAP. TNAP screening was developed and performed at the Sanford-Burnham Center for Chemical Genomics (SBCCG) as part of the Molecular Library Screening Center Network (MLSCN). XO1 submission, MH077602-01, Pharmacological inhibitors o
- Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay - Set 2 Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response concentration confirmation of uHTS hits from a small molecule activators of human intestinal alkaline phosphatase via a luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response concentration confirmation of uHTS hits from a small molecule activators of mouse intestinal alkaline phosphatase via a luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of IAP is unknown. The goal of this HTS is to confirm hits in "uHTS Luminescent assay for identificatio
- Dose Response confirmation of uHTS activators of Human Intestinal Alkaline Phosphatase using Mouse Intestinal Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of IAP is unknown. The goal of this MLPCN probe project is to identify novel and specific activators of
- Dose Response confirmation of uHTS activators of Human Intestinal Alkaline Phosphatase using Placental Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS activators of Human Intestinal Alkaline Phosphatase using Tissue Nonspecific Alkaline Phosphatase. Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS activators of Mouse Intestinal Alkaline Phosphatase using Human Intestinal Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS activators of Mouse Intestinal Alkaline Phosphatase using Placental Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of IAP is unknown. The goal of this HTS is to confirm hits in "uHTS Luminescent assay for identificatio
- Dose Response confirmation of uHTS activators of Mouse Intestinal Alkaline Phosphatase using Tissue Nonspecific Alkaline Phosphatase. Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of IAP is unknown. The goal of this HTS is to confirm hits in "uHTS Luminescent assay for identificatio
- Dose Response confirmation of uHTS hits from a small molecule inhibitors of human intestinal alkaline phosphatase via a luminescent assay - Set 2 Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological functio
- Dose Response confirmation of uHTS hits from a small molecule inhibitors of human intestinal alkaline phosphatase via a luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS hits from a small molecule inhibitors of mouse intestinal alkaline phosphatase via a luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS inhibitors of Human Intestinal Alkaline Phosphatase using Mouse Intestinal Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS inhibitors of Human Intestinal Alkaline Phosphatase using Placental Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of I
- Dose Response confirmation of uHTS inhibitors of Human Intestinal Alkaline Phosphatase using Tissue Nonspecific Alkaline Phosphatase. Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological functio
- Dose Response confirmation of uHTS inhibitors of Mouse Intestinal Alkaline Phosphatase using Human Intestinal Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological functio
- Dose Response confirmation of uHTS inhibitors of Mouse Intestinal Alkaline Phosphatase using Placental Alkaline Phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Dose Response confirmation of uHTS inhibitors of Mouse Intestinal Alkaline Phosphatase using Tissue Nonspecific Alkaline Phosphatase. Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Proposal Number: X01-MH077602-01 Assay Provider Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute(SBMRI, San Diego, CA) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. The exact biological function of
- Luminescent assay for HTS discovery of chemical inhibitors of placental alkaline phosphatase confirmation Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Proposal Number: MH077602-01 Assay Provider: Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute, San Diego, CA. This PLAP dose response assay is developed and performed to confirm hits originally identified in the PLAP Luminescent HTS assay (AID 690) and to study the structure-activity relationship on analogs of the confirmed hits. Compounds are either acquired from commercial sources or synthesized internally. Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified: three isozymes are tissue-specific and the fourth one is tissue-nonspecific. Placental alkaline phosphatase (PLAP) is high
- Luminescent assay for identification of inhibitors of human intestinal alkaline phosphatase Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Proposal Number: XO1 MH077602-01 Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing inorganic phosphate and alcohol. APs are dimeric enzymes found in most organisms. In human, four isozymes of APs have been identified. One isozyme is tissue-nonspecific (designated TNAP) and three other isozymes are tissue-specific and named according to the tissue of their predominant expression: intestinal (IAP), placental (PLAP) and germ cell (GCAP) alkaline phosphatases. IAP expression is largely restricted to the gut, especially to the epithelial cells (enterocytes) of the small intestinal mucosa. IAP is inhibited by a number of inhibitors (1). They include L-phenylalanine, (2, 3), L-tryptophan (4), L-leucine and phenylalanine-g
- SAR assay for compounds activating TNAP in the absence of phosphate acceptor performed in a luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Proposal Number: 1R03 MH082385-01 This TNAP dose response assay is developed and performed to confirm hits originally identified in the TNAP luminescent HTS assay (AID 1001) and to study the structure-activity relationship on analogs of the confirmed hits. Compounds are either acquired from commercial sources or synthesized internally. Alkaline phosphatases (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in the most organisms. In human, four isozymes of APs have been identified. Three isozymes are tissue-specific and the fourth one is tissue-non specific, named TNAP. TNAP deficiency is associated with defective bone mineralization in the form of rickets and osteomalacia. Therefore, t
- SAR assay for compounds activating TNAP in the presence of 100 mM DEA performed in a luminescence assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Proposal Number: 1R03 MH082385-01 This TNAP dose response assay is developed and performed to confirm hits originally identified in the TNAP luminescent HTS assay (AID 1001) and to study the structure-activity relationship on analogs of the confirmed hits. Compounds are either acquired from commercial sources or synthesized internally. Alkaline phosphatases (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in the most organisms. In human, four isozymes of APs have been identified. Three isozymes are tissue-specific and the fourth one is tissue-non specific, named TNAP. TNAP deficiency is associated with defective bone mineralization in the form of rickets and osteomalacia. Therefore, t
- SAR assay for compounds inhibiting TNAP in the absence of phosphate acceptor performed in a luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Number: MH077602-01 Assay Provider: Dr. Jose Luis Millan, Sanford-Burnham Medical Research Institute, San Diego, CA This TNAP dose response assay is developed and performed for the purpose of SAR study on analogs of hits originally identified in the TNAP luminescent HTS assay (AID 518) Alkaline phosphatase (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in the most organism. In human, four isozymes of APs have been identified. Three isozymes are tissue-specific and the fourth one is tissue-nonsepecifc, named TNAP. TNAP overexpression is associated with excessive calcification observed in different tissues. Therefore, there are therapeutic potentials of in
- uHTS identification of compounds activating TNAP in the absence of phosphate acceptor performed in luminescent assay Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Screening Centers Network (MLSCN) Grant Proposal Number: 1R03 MH082385-01 Alkaline phosphatases (EC 3.1.3.1) (APs) catalyze the hydrolysis of phosphomonoesters, releasing phosphate and alcohol. APs are dimeric enzymes found in the most organisms. In human, four isozymes of APs have been identified. Three isozymes are tissue-specific and the fourth one is tissue-non specific, named TNAP. TNAP deficiency is associated with defective bone mineralization in the form of rickets and osteomalacia. Therefore, there is therapeutic potential of modulating TNAP activity. The goal of this HTS is to identify novel and specific activators of TNAP. The only known to date class of alkaline phosphatases activators are amino-containing alcohols, such as diethanolamine (DEA), that act as phosphoacceptor substrate and exh
- Biochemical assay Biochemical assays were performed using a Biotek Synergy H1 microplate reader. Prior to their evaluation, initial experiments were performed to confirm the synthesized analogues do not inhibit the coupling enzymes utilized in the substrate and inhibition assays. Metabolomics: LC gradient was employed at a flow rate of 200 μL/min on an Agilent 1150 LC system (Agilent, Santa Clara, Calif., USA); mass spectrometry was performed on a Q-Exactive mass spectrometer (Thermo Fisher Scientific, Waltham, Mass., USA). Crystallographic data were collected on beamlines 23ID-B and 23ID-D of GM/CA@APS of the Advanced Photon Source (APS) using X-rays of 0.99 Å wavelength and Rayonix (formerly MAR-USA) 4×4 tiled CCD detector with a 300 mm2 sensitive area.
- Determination of Dissociation Constant (Kd) Values of Ki were determined from the dependence of the observed rate constant (kobs) on inhibitor concentration. With subsaturing APS, the inhibition constant is equal to the dissociation constant (Ki = Kd). A range of inhibitor concentrations was employed from at least 5-fold below to 10-fold above the inhibition constant. Nonlinear least-squares fits of the equation for competitive inhibition gave excellent fits in all cases, and the standard error was typically less than 15%.
- QFRET-based biochemical high throughput dose response assay for inhibitors of the Plasmodium falciparum M18 Aspartyl Aminopeptidase (PFM18AAP). Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: John Dalton and Donald Gardiner, Queensland Institute of Medical Research, Australia Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: 1 R03 MH084103-01 Grant Proposal PI: John Dalton and Donald Gardiner, Queensland Institute of Medical Research, Australia External Assay ID: PFM18AAP_INH_QFRET_1536_3XIC50 Name: QFRET-based biochemical high throughput dose response assay for inhibitors of the Plasmodium falciparum M18 Aspartyl Aminopeptidase (PFM18AAP). Description: Aminopeptidases (APs) are metalloproteases that cleave amino-terminal (N-terminal) amino acids during protein synthesis (1, 2) These enzymes are characterized in part by their post-translational removal of leucine, aspartate, proline, methionine, etc from proteins and peptides, in order that proteins are properly regulated, targete
- Counterscreen for inhibitors of M1 and M17 aminopeptidases: QFRET-based biochemical high throughput dose response assay for inhibitors of the Cathepsin L proteinase (CTSL1). Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: John Dalton and Donald Gardiner, Queensland Institute of Medical Research, Australia Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: 1 R03 MH084103-01 Grant Proposal PI: John Dalton and Donald Gardiner, Queensland Institute of Medical Research, Australia External Assay ID: CTSL1_INH_QFRET_1536_3XIC50 CSDRUN Name: Counterscreen for inhibitors of M1 and M17 aminopeptidases: QFRET-based biochemical high throughput dose response assay for inhibitors of the Cathepsin L proteinase (CTSL1). Description: Aminopeptidases (APs) are metalloproteases that cleave amino-terminal (N-terminal) amino acids during protein synthesis (1, 2) These enzymes are characterized in part by their post-translational removal of leucine, aspartate, proline, methionine, etc from proteins and peptides, in order that protei
- QFRET-based counterscreen for inhibitors of PFM18AAP: biochemical high throughput dose response assay for inhibitors of the Cathepsin L proteinase (CTSL1). Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provider: John Dalton and Donald Gardiner, Queensland Institute of Medical Research, Australia Network: Molecular Library Probe Production Centers Network (MLPCN) Grant Proposal Number: 1 R03 MH084103-01 Grant Proposal PI: John Dalton and Donald Gardiner, Queensland Institute of Medical Research, Australia External Assay ID: CTSL1_INH_QFRET_1536_3XIC50 Name: QFRET-based counterscreen for inhibitors of PFM18AAP: biochemical high throughput dose response assay for inhibitors of the Cathepsin L proteinase (CTSL1). Description: Aminopeptidases (APs) are metalloproteases that cleave amino-terminal (N-terminal) amino acids during protein synthesis (1, 2) . These enzymes are characterized in part by their post-translational removal of leucine, aspartate, proline, methionine, etc from proteins and peptides, in order that proteins are properly regulat
 Apatinib BDBM50152828 YN-968D1
Apatinib BDBM50152828 YN-968D1 APS-3-77 US10548897, Compound 50 BDBM429415
APS-3-77 US10548897, Compound 50 BDBM429415 US10548897, Compound 10 APS-2-12 BDBM429418
US10548897, Compound 10 APS-2-12 BDBM429418 US10548897, Compound 21 BDBM429286 APS-2-79
US10548897, Compound 21 BDBM429286 APS-2-79 US10548897, Compound 32 BDBM429421 APS-3-6
US10548897, Compound 32 BDBM429421 APS-3-6 US10548897, Compound 11 BDBM429420 D3RKN_87 APS-2-16
US10548897, Compound 11 BDBM429420 D3RKN_87 APS-2-16 Adenosine Phosphosulfate (APS) BDBM25461 Adenosine 5-Phosphosulfate Adenylyl sulfate [({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]sulfonic acid
Adenosine Phosphosulfate (APS) BDBM25461 Adenosine 5-Phosphosulfate Adenylyl sulfate [({[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy]sulfonic acid