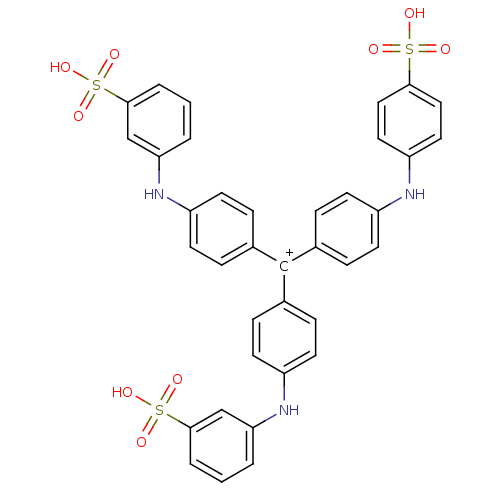
 BHT, 1 BDBM29329
BHT, 1 BDBM29329 2,6-di-tert-butyl-4-methylphenol Butylhydroxytoluene 2,6-Di-tert-butyl-p-cresol 2,6-Di-tert-butyl-1-hydroxy-4-methylbenzene BDBM50079507 Butylated hydroxytoluene CHEMBL146 2,6-Di-t-butyl-4-methylphenol 2,6-Di-tert-butyl-4-cresol 2,6-Bis(1,1-dimethylethyl)-4-methylphenol BHT
2,6-di-tert-butyl-4-methylphenol Butylhydroxytoluene 2,6-Di-tert-butyl-p-cresol 2,6-Di-tert-butyl-1-hydroxy-4-methylbenzene BDBM50079507 Butylated hydroxytoluene CHEMBL146 2,6-Di-t-butyl-4-methylphenol 2,6-Di-tert-butyl-4-cresol 2,6-Bis(1,1-dimethylethyl)-4-methylphenol BHT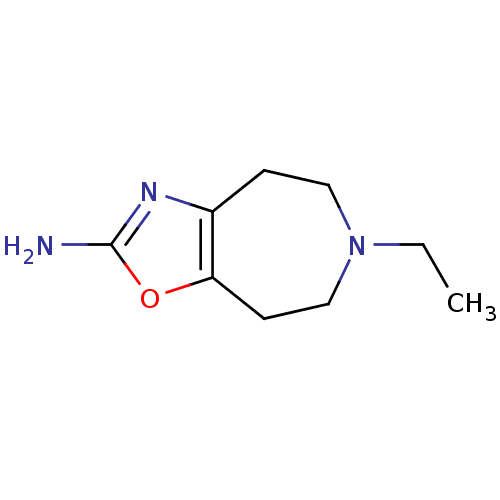 CAS_128-37-0 NSC_169743 BHT-933 BDBM85676
CAS_128-37-0 NSC_169743 BHT-933 BDBM85676 2-TERT-BUTYL-4-HYDROXYANISOLE BDBM50377940 BHA Butylated hydroxyanisole
2-TERT-BUTYL-4-HYDROXYANISOLE BDBM50377940 BHA Butylated hydroxyanisole Talipexole BDBM50026975 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine (B-HT 920) 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine(BHT 920) CHEMBL279085
Talipexole BDBM50026975 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine (B-HT 920) 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine(BHT 920) CHEMBL279085
- Cai, P; Fang, SQ; Yang, HL; Yang, XL; Liu, QH; Kong, LY; Wang, XB Donepezil-butylated hydroxytoluene (BHT) hybrids as Anti-Alzheimer's disease agents with cholinergic, antioxidant, and neuroprotective properties. Eur J Med Chem 157: 161-176 (2018)
- Luo, Z; Li, S; Zhang, Y; Yin, F; Luo, H; Chen, X; Cui, N; Wan, S; Li, X; Kong, L; Wang, X Oxazole-4-carboxamide/butylated hydroxytoluene hybrids with GSK-3β inhibitory and neuroprotective activities against Alzheimer's disease. Eur J Med Chem 256: (2023)
- In Vitro Radioactive (RTA) Assay An in vitro assay for determining ICso's to identify compounds that inhibit CETP transfer activity is performed based on a modification of a published method (Morton and Zilversmit, (1981) A plasma inhibitor of triglyceride and cholesteryl ester transfer activities, J. Biol. Chem. 256(23), 1 1992-1 1995). The ability of inhibitors to alter CETP activity is performed using two different assays: one using recombinant CETP and one using an endogenous plasma source of CETP. Both assays measure the transfer of [3H] cholesteryl oleate or [3H] triolein from exogenous LDL to HDL.Radiolabeled donor particles are generated by first combining 100 ul of 200 uM butylated hydroxyl toluene in CHC13, 216 of 21.57 mM DOPC in EtOH, and either 500 uCi [3H]-triolein (Perkin Elmer #NET-431) or 500 uCi [3H]-cholesteryl oleate (GE #TRK886) in a glass tube. Reagents are mixed, dried under nitrogen, and then resuspended in 2 mL of 50 mM Tris, 27 uM EDTA.
- In Vitro Radioactive Assays of CETP-Catalyzed CE and TG Transfer (RTA Assay) Radiolabeled donor particles are generated by first combining 100 μl of 200 μM butylated hydroxyl toluene in CHCl3, 216 μL of 21.57 mM DOPC in EtOH, and either 500 μCi [3H]-triolein (Perkin Elmer #NET-431) or 500 μCi [3H]-cholesteryl oleate (GE #TRK886) in a glass tube. Reagents are mixed, dried under nitrogen, and then resuspended in 2 mL of 50 mM Tris, 27 μM EDTA at pH 7.4. After a brief vortex, the solution is sonicated until clear and mixed with 20 mL of fresh human serum. The mixture is incubated overnight at 37° C. The [3H] labeled LDL substrate is separated at 1.063 g/ml density by sequential ultracentrifugal flotation in NaBr according to the method by (Havel, Eder et al. 1955; Chapman, Goldstein et al. 1981). Once isolated the particles are dialyzed 3× in CETP buffer (50 mM Tris, pH 7.4, 100 mM NaCl, 1 mM EDTA). Human HDL is purchased from Intracel and used as the acceptor particles. Transfer assays are performed in a 96 or 384-well v-bottom polypropylene plate. For the RTA using recombinant CETP (2% RTA), an assay cocktail is prepared with the final concentrations 128 μg/mL HDL, 20 nM rCETP, 2% human serum, and 1×CETP buffer. 1 μL of each test compound diluted in DMSO is added to 47 μL of assay cocktail per well and incubated at 37° C. for 1 hour. To initiate the transfer reaction, 2 μL radiolabeled LDL is added. After an additional 60 min of incubation at 37° C., the transfer action is terminated by precipitation of LDL with an equal volume of 20% W/V PEG 8000. The plates are centrifuged at 2000 rpm for 30 minutes at 4° C. A 40 μL aliquot of the HDL-containing supernatant is transferred to a Packard Optiplate™ with 200 μL of MicroScint™ 20. After mixing, plates are counted by liquid scintillation.
 BHT, 1 BDBM29329
BHT, 1 BDBM29329 2,6-di-tert-butyl-4-methylphenol Butylhydroxytoluene 2,6-Di-tert-butyl-p-cresol 2,6-Di-tert-butyl-1-hydroxy-4-methylbenzene BDBM50079507 Butylated hydroxytoluene CHEMBL146 2,6-Di-t-butyl-4-methylphenol 2,6-Di-tert-butyl-4-cresol 2,6-Bis(1,1-dimethylethyl)-4-methylphenol BHT
2,6-di-tert-butyl-4-methylphenol Butylhydroxytoluene 2,6-Di-tert-butyl-p-cresol 2,6-Di-tert-butyl-1-hydroxy-4-methylbenzene BDBM50079507 Butylated hydroxytoluene CHEMBL146 2,6-Di-t-butyl-4-methylphenol 2,6-Di-tert-butyl-4-cresol 2,6-Bis(1,1-dimethylethyl)-4-methylphenol BHT CAS_128-37-0 NSC_169743 BHT-933 BDBM85676
CAS_128-37-0 NSC_169743 BHT-933 BDBM85676 2-TERT-BUTYL-4-HYDROXYANISOLE BDBM50377940 BHA Butylated hydroxyanisole
2-TERT-BUTYL-4-HYDROXYANISOLE BDBM50377940 BHA Butylated hydroxyanisole Talipexole BDBM50026975 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine (B-HT 920) 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine(BHT 920) CHEMBL279085
Talipexole BDBM50026975 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine (B-HT 920) 6-Allyl-5,6,7,8-tetrahydro-4H-thiazolo[4,5-d]azepin-2-ylamine(BHT 920) CHEMBL279085