Query String: Chloroquine amodiaquine
 CHLOROQUINE DIPHOSPHATE CHLOROQUINE PHOSPHATE BDBM50411863 ARALEN
CHLOROQUINE DIPHOSPHATE CHLOROQUINE PHOSPHATE BDBM50411863 ARALEN DESETHYLCHLOROQUINE Desethyl chloroquine BDBM50408762
DESETHYLCHLOROQUINE Desethyl chloroquine BDBM50408762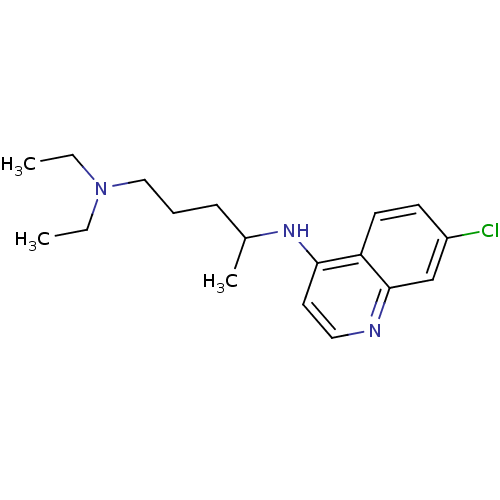 BDBM22985 Chlorochin CHLOROQUINE PHOSPHATE Chloroquine Chloroquine, 17 Aralen med.21724, Compound 8 {4-[(7-chloroquinolin-4-yl)amino]pentyl}diethylamine CHEMBL76
BDBM22985 Chlorochin CHLOROQUINE PHOSPHATE Chloroquine Chloroquine, 17 Aralen med.21724, Compound 8 {4-[(7-chloroquinolin-4-yl)amino]pentyl}diethylamine CHEMBL76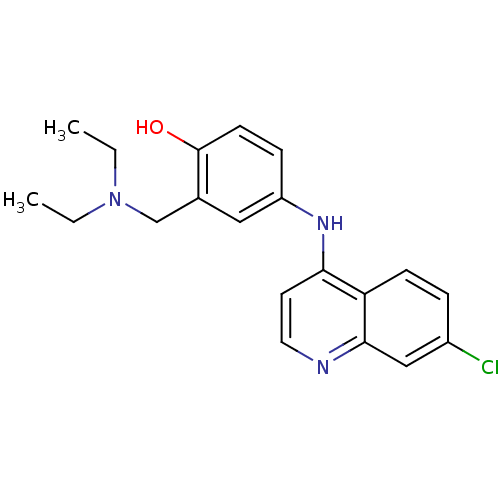 4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)methyl]phenol AMODIAQUINE BDBM50041457 med.21724, Compound 188 CHEMBL682
4-[(7-chloroquinolin-4-yl)amino]-2-[(diethylamino)methyl]phenol AMODIAQUINE BDBM50041457 med.21724, Compound 188 CHEMBL682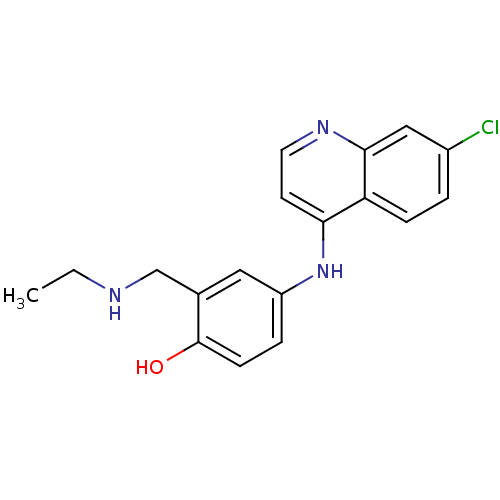 CHEMBL1235 N-Monodesethylamodiaquine (Deaq) desethylamodiaquine 4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl-phenol BDBM50056190 Monodesethylamodiaquine bidesethylamodiaquine Desethyl amodiaquine
CHEMBL1235 N-Monodesethylamodiaquine (Deaq) desethylamodiaquine 4-(7-Chloro-quinolin-4-ylamino)-2-ethylaminomethyl-phenol BDBM50056190 Monodesethylamodiaquine bidesethylamodiaquine Desethyl amodiaquine
- Stocks, PA; Raynes, KJ; Bray, PG; Park, BK; O'Neill, PM; Ward, SA Novel short chain chloroquine analogues retain activity against chloroquine resistant K1 Plasmodium falciparum. J Med Chem 45: 4975-83 (2002)
- Zishiri, VK; Joshi, MC; Hunter, R; Chibale, K; Smith, PJ; Summers, RL; Martin, RE; Egan, TJ Quinoline antimalarials containing a dibemethin group are active against chloroquinone-resistant Plasmodium falciparum and inhibit chloroquine transport via the P. falciparum chloroquine-resistance transporter (PfCRT). J Med Chem 54: 6956-68 (2011)
- Deane, KJ; Summers, RL; Lehane, AM; Martin, RE; Barrow, RA Chlorpheniramine Analogues Reverse Chloroquine Resistance in Plasmodium falciparum by Inhibiting PfCRT. ACS Med Chem Lett 5: 576-81 (2014)
- Pérez, BC; Fernandes, I; Mateus, N; Teixeira, C; Gomes, P Recycling antimalarial leads for cancer: Antiproliferative properties of N-cinnamoyl chloroquine analogues. Bioorg Med Chem Lett 23: 6769-72 (2013)
- Biot, C; Daher, W; Ndiaye, CM; Melnyk, P; Pradines, B; Chavain, N; Pellet, A; Fraisse, L; Pelinski, L; Jarry, C; Brocard, J; Khalife, J; Forfar-Bares, I; Dive, D Probing the role of the covalent linkage of ferrocene into a chloroquine template. J Med Chem 49: 4707-14 (2006)
- Lee, SG; Alpert, TD; Jez, JM Crystal structure of phosphoethanolamine methyltransferase from Plasmodium falciparum in complex with amodiaquine. Bioorg Med Chem Lett 22: 4990-3 (2012)
- Vippagunta, SR; Dorn, A; Matile, H; Bhattacharjee, AK; Karle, JM; Ellis, WY; Ridley, RG; Vennerstrom, JL Structural specificity of chloroquine-hematin binding related to inhibition of hematin polymerization and parasite growth. J Med Chem 42: 4630-9 (1999)
- Solaja, BA; Opsenica, D; Smith, KS; Milhous, WK; Terzić, N; Opsenica, I; Burnett, JC; Nuss, J; Gussio, R; Bavari, S Novel 4-aminoquinolines active against chloroquine-resistant and sensitive P. falciparum strains that also inhibit botulinum serotype A. J Med Chem 51: 4388-91 (2008)
- Relitti, N; Federico, S; Pozzetti, L; Butini, S; Lamponi, S; Taramelli, D; D'Alessandro, S; Martin, RE; Shafik, SH; Summers, RL; Babij, SK; Habluetzel, A; Tapanelli, S; Caldelari, R; Gemma, S; Campiani, G Synthesis and biological evaluation of benzhydryl-based antiplasmodial agents possessing Plasmodium falciparum chloroquine resistance transporter (PfCRT) inhibitory activity. Eur J Med Chem 215: (2021)
- Miura, T; Hidaka, K; Azai, Y; Kashimoto, K; Kawasaki, Y; Chen, SE; de Freitas, RF; Freire, E; Kiso, Y Optimization of plasmepsin inhibitor by focusing on similar structural feature with chloroquine to avoid drug-resistant mechanism of Plasmodium falciparum. Bioorg Med Chem Lett 24: 1698-701 (2014)
- Chipeleme, A; Gut, J; Rosenthal, PJ; Chibale, K Synthesis and biological evaluation of phenolic Mannich bases of benzaldehyde and (thio)semicarbazone derivatives against the cysteine protease falcipain-2 and a chloroquine resistant strain of Plasmodium falciparum. Bioorg Med Chem 15: 273-82 (2007)
- O'Neill, PM; Shone, AE; Stanford, D; Nixon, G; Asadollahy, E; Park, BK; Maggs, JL; Roberts, P; Stocks, PA; Biagini, G; Bray, PG; Davies, J; Berry, N; Hall, C; Rimmer, K; Winstanley, PA; Hindley, S; Bambal, RB; Davis, CB; Bates, M; Gresham, SL; Brigandi, RA; Gomez-de-Las-Heras, FM; Gargallo, DV; Parapini, S; Vivas, L; Lander, H; Taramelli, D; Ward, SA Synthesis, antimalarial activity, and preclinical pharmacology of a novel series of 4'-fluoro and 4'-chloro analogues of amodiaquine. Identification of a suitable"back-up" compound for N-tert-butyl isoquine. J Med Chem 52: 1828-44 (2009)
- ChEMBL_769822 (CHEMBL1831816) Inhibition of chloroquine-resistant Plasmodium falciparum Dd2 chloroquine resistance transporter expressed in xenopus laevis oocytes assessed as inhibition of [3H]chloroquine uptake after 1 to 2 hrs
- ChEMBL_877808 (CHEMBL2187303) Inhibition of chloroquine-resistant Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocytes assessed as reduction in [3H]-chloroquine uptake after 1.5 to 2 hrs
- ChEMBL_864478 (CHEMBL2176281) Inhibition of chloroquine-resistant Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocyte assessed as inhibition of [3H]chloroquine uptake measured from 1 to 2 hrs
- ChEMBL_1435702 (CHEMBL3388335) Inhibition of CYP2C8 (unknown origin) using amodiaquine substrate
- ChEMBL_1364181 (CHEMBL3292896) Inhibition of chloroquine-resistant Plasmodium falciparum Dd2 CRT expressed in Xenopus laevis oocytes plasma membrane assessed as reduction of [3H]-chloroquine transportation after 1 to 2 hrs
- ChEMBL_692839 (CHEMBL1637353) Inhibition of human CYP2C8 using amodiaquine as a substrate
- ChEBML_1683158 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate
- ChEMBL_1667013 (CHEMBL4016809) Inhibition of human liver microsomes CYP2C8 using amodiaquine as substrate
- ChEMBL_584109 (CHEMBL1051116) Inhibition of hemozoin formation after 48 hrs in chloroquine-sensitive Plasmodium falciparum NF54
- ChEMBL_1981694 (CHEMBL4614956) Inhibition of CYP2C8 (unknown origin) assessed as reduction in amodiaquine N-deethylation
- ChEMBL_2100789 (CHEMBL4809185) Direct inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate
- ChEMBL_2265891 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS analysis
- ChEMBL_2282759 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_2290734 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_1824050 (CHEMBL4323814) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_1825000 (CHEMBL4324764) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_1832034 (CHEMBL4332042) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_1979600 (CHEMBL4612735) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_2022413 (CHEMBL4676226) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_2110155 (CHEMBL4818830) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_2165290 (CHEMBL5050151) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_2200574 (CHEMBL5113090) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis
- ChEMBL_1526170 (CHEMBL3636695) Reversible inhibition of CYP2C8 amodiaquine-N-deethylase activity in human liver microsomes by LC-MS/MS analysis
- ChEMBL_2100799 (CHEMBL4809195) Time-dependent inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 30 mins
- ChEMBL_2252748 (CHEMBL5166958) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate in presence or absence of NADPH
- ChEBML_1695898 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate after 10 mins by LC-MS/MS analysis
- ChEMBL_2278018 Inhibition of CYP2C8 in human liver microsome suspension using amodiaquine substrate incubated for 30 mins by LC/MS analysis
- ChEMBL_1486160 (CHEMBL3533279) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate after 5 mins by LC-MS/MS analysis
- ChEMBL_2296117 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate in presence of NADPH by LC-MS/MS analysis
- ChEMBL_1904192 (CHEMBL4406414) Inhibition of CYP2C8 in human pooled liver microsomes using amodiaquine as substrate after 10 mins by UPLC-MS/MS analysis
- ChEMBL_2030249 (CHEMBL4684407) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate incubated for 30 mins by LC-MS/MS analysis
- ChEMBL_2035469 (CHEMBL4689627) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate incubated for 15 mins by LC-MS/MS analysis
- ChEMBL_2073304 (CHEMBL4728838) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate measured after 15 mins by LC-MS/MS analysis
- ChEMBL_2101773 (CHEMBL4810169) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate substrate in presence of NADPH by LC-MS/MS analysis
- ChEMBL_1658365 (CHEMBL4007977) Inhibition of microsomal CYP2C8 (unknown origin) using amodiaquine as substrate preincubated for 5 mins followed by NADPH addition by LC-MS/MS analysis
- ChEMBL_1920112 (CHEMBL4422957) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate after 15 mins in presence of NADPH by LC-MS/MS analysis
- ChEMBL_2209733 (CHEMBL5122682) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated with compound followed by NADPH addition by LC-MS/MS analysis
- In Vitro Inhibition Assay In vitro antimalarial assay, antimalarial activity of the compounds was determined in vitro on chloroquine sensitive and resistant strains of plasmodium flaciparum as described earlier.
- ChEMBL_1586832 (CHEMBL3826664) Inhibition of CYP2C8 in human liver microsomes in presence of NADPH using amodiaquine as substrate measured within 2.5 mins by LC-MS/MS analysis
- ChEMBL_1742656 (CHEMBL4158406) Inhibition of of CYP2C8 in human liver microsomes using amodiaquine as substrate after 10 mins in presence of NADPH by LC-MS/MS analysis
- ChEMBL_2291487 Inhibition of CYP2C8 in pooled human liver microsomes using amodiaquine as substrate assessed as reduction in N-desethylamodiaquine metabolite formation by LC-MS/MS analysis
- ChEMBL_2277113 Inhibition of CYP2C8 (unknown origin) using amodiaquine as substrate incubated for 10 to 20 mins in presence of NADPH-generating system by LC-MS/MS analysis
- ChEMBL_2017917 (CHEMBL4671495) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate incubated for 15 mins in presence of NADPH generating system by LC-MS/MS analysis
- ChEMBL_2194838 (CHEMBL5107198) Inhibition of CYP2C8 in human liver microsomes at 10 uM using amodiaquine as substrate preincubated for 10 mins followed by NADPH addition by LC/MS analysis
- ChEMBL_1770954 (CHEMBL4223066) Inhibition of falcipain-2 in chloroquine resistant Plasmodium falciparum RKL9 schizont stage infected in human erythrocytes assessed as reduction in bacterial growth after 24 hrs by Giemsa staining based assay
- ChEMBL_823394 (CHEMBL2046053) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate incubated for 3 mins prior to NAPDH-addition measured after 30 mins by RP-HPLC analysis
- ChEMBL_1770953 (CHEMBL4223065) Inhibition of falcipain-2 in chloroquine sensitive Plasmodium falciparum MRC-02 schizont stage infected in human erythrocytes assessed as reduction in bacterial growth after 24 hrs by Giemsa staining based assay
- ChEBML_1688704 Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 15 mins measured after 8 mins in presence or absence of NADPH by LC/MS/MS analysis
- ChEMBL_2248277 (CHEMBL5162487) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as a substrate preincubated for 10 mins followed by NADPH addition and measured after 10 mins by LC/MS/MS analysis
- ChEMBL_1735367 (CHEMBL4150903) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 5 mins followed by addition of NADPH-regenerating system and measured after 15 mins by UPLC-MS analysis
- ChEMBL_1845026 (CHEMBL4345453) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 to 30 mins by LC-MS/MS analysis
- ChEMBL_1902236 (CHEMBL4404458) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 5 mins followed by NADPH addition and measured for 10 to 30 mins by LC-MS/MS analysis
- ChEMBL_1904187 (CHEMBL4406409) Inhibition of CYP2C8 in human pooled liver microsomes using amodiaquine as substrate preincubated with NADPH for 30 mins followed by substrate addition and measured after 10 mins by UPLC-MS/MS analysis
- ChEMBL_2080679 (CHEMBL4736470) Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 10 mins in presence of NADPH generating system followed by substrate addition and measured after 10 mins by LC-MS/MS analysis
- Cytochrome P450 2C9 Inhibition Assay (Amodiaquine) The inhibition of cytochrome P450 2C8-isoenzyme catalysed deethylation of Amodiaquine by the test compound is assayed at 37° C. with human liver microsomes. All assays are carried out on a robotic system in 96 well plates. The final incubation volume contains TRIS buffer (0.1 M), MgCl2 (5 mM), human liver microsomes (0.05 mg/ml), Amodiaquine (1 uM) and the test compound at five different concentrations or no compound (high control) in duplicate (e.g. highest concentration 10-50 uM with subsequent serial 1:4 dilutions). Following a short preincubation period, reactions are started with the cofactor (NADPH, 1 mM) and stopped by cooling the incubation down to 8° C. and subsequently by addition of one volume of acetonitrile.
- Inhibition Assays The final incubation volume contains TRIS buffer (0.1 M), MgCl2 (5 mM), a certain concentration of human liver microsomes dependent on the P450 isoenzyme measured (P450 2C9, P450 3A4: 0.1 mg/ml; P450 2D6: 0.2 mg/ml; P450 2C19: 0.5 mg/ml; P450 2C8: 0.05 mg/ml) and a certain concentration of the individual substrate for each isoenzyme (P450 2C9: Diclofenac 10 μM; P450 3A4: Midazolam 5 μM; P450 2D6: Dextromethorphan 5 μM; P450 2C19: S-Mephenytoin 70 μM; P450 2C8: Amodiaquine 1 μM).
- Inhibition of CYP-2C8 The inhibition of cytochrome P450 2C8-isoenzyme catalyzed deethylation of amodiaquine by the test compound is assayed at 37° C. with human liver microsomes. All assays are carried out on a robotic system in 96 well plates. The final incubation volume contains TRIS buffer (0.1 M), MgCl2 (5 mM), human liver microsomes (0.05 mg/mL), amodiaquine (1 μM) and the test compound at five different concentrations or no compound (high control) in duplicate (e.g. highest concentration 10-50 μM with subsequent serial 1:4 dilutions). Following a short preincubation period, reactions are started with the cofactor (NADPH, 1 mM) and stopped by cooling the incubation down to 8° C. and subsequently by addition of one volume of acetonitrile. An internal standard solution the stable isotope d5-desethylamodiaquine is added after quenching of incubations. Peak area analyte (=metabolite formed) and internal standard is determined by LC-MS/MS. The resulting peak area ratio analyte to internal standard in these incubations is compared to a control activity containing no test compound. Within each of the assay runs, the IC50 of a positive control inhibitor (Montelukast) is determined. Experimental IC50 values are calculated by least square regression according to the following equation: % control activity=(100% control activity/(1+(I/IC 50)S))−B with I=inhibitor concentration; S=slope factor; B=background activity (lower plateau of the inhibition curve).
- CYP Enzyme Inhibition Assay Human liver microsomes (HLMs) (0.3 mg/mL in 0.1-M potassium phosphate buffer, pH 7.4) are incubated with CYP (cytochromes P450) isozyme-selective substrates (phenacetin for CYP1A2, amodiaquine for CYP2C8, diclofenac for CYP2C9, S-mephenytoin for CYP2C19, Dextromethorphan for CYP2D6, and midalozam for CYP3A4/5), and multiple concentrations of Compound (I-1) or Ko143 (0, 0.041, 0.12, 0.37, 1.11, 3.33, 10, and 30 uM) in 96-well plates. Reactions are initiated by the addition of β-NADPH (2 mM) and MgCl2 (3 mM) in 0.1-M potassium phosphate buffer, pH 7.4. Reactions are incubated for 12 minutes at 37° C., and then terminated by the addition of an equal volume of ACN containing 1-uM carbutamide (IS). The plates are refrigerated at approximately 4° C. for 15 minutes and then centrifuged in order to pellet the precipitated proteins.
- Growth Inhibition Assay The [3H]-hypoxanthine growth inhibition assay (Desjardins et al., 1979 Antimicrobial Agents Chemother 16: 710-718) was used to evaluate the in vitro antimalarial activity of the compounds. Briefly, synchronised parasite cultures (>90% rings, 6 to 8 h post invasion) in [3H]-RPMI-LPLF complete medium with 1% parasitaemia and 2% haematocrit were exposed to the compounds at ten two-fold concentrations. Chloroquine was used as a reference drug. Uninfected RBCs at 2% haematocrit were used as background controls. Two drug exposure periods were evaluated (48 h and 96 h). For the 48 h exposure period, the plates were incubated in the gas mixture at 37° C. for approximately 20 h (about 24 h post-invasion). To each well, 0.2 uCi of tritiated hypoxanthine (GE Healthcare, Amersham) solution in [3H]RPMI-1640-LPLF was added. The plates were incubated for a further 24 h at 37° C. in the gas Mixture and then frozen at 20° C. For the 96 h exposure period, the plates were incubated.
- CYP Enzyme Inhibitory Activity IC50 Assay The CYP enzyme probe substrates used in the experiment were: Phenacetin (1A2), Bupropion (2B6), Amodiaquine (2C8), Mephenytoin (2C19), Diclofenac (2C9), Dextromethorphan (2D6) and Testosterone (3A4/5). The final concentration of microsomes in the experimental system was 0.1 mg/mL. PBS Buffer was 50 mM K2HPO4 buffer. The concentrations of the compound to be tested were 50 μM, 12.5 μM, 3.125 μM, 0.781 μM, 0.195 μM, and 0.0488 μM, respectively. The corresponding probe substrates and microsomes were added into PBS, mixed well and dispensed into each reaction system, then control compound/compound to be tested/DMSO solution was added into the corresponding reaction systems respectively. The reaction system was mixed well, pre-incubated in a water bath at 37° C. for 5 minutes, added with 10 mM NADPH solution and mixed well, and reacted in a water bath at 37° C. for 10 minutes. After the reaction was completed, an internal standard acetonitrile solution was added to terminate the reaction. Centrifugation was performed at 4000 rpm, and the supernatant solution was taken and mixed well with an equal volume of pure water.
- Parasite Proliferation Assay This parasite proliferation assay, a modification of published DNA intercalating fluorescent dye-based assay, was adapted to 384-well plate format and measures the increase in parasite DNA content using the DNA intercalating dye SYBR Green. Compound 1 exhibited strong potency in both the in vitro biochemical and parasite proliferation assays, which suggests that this compound is potentially acting against PfCDPK1 in vivo. Several other analogs, compounds 7 and 8, exhibited potent enzymatic activity but were inactive in parasite proliferation assays, whereas some analogs displayed low EC50 values in the parasite proliferation assay but were inactive against the enzyme. The EC50 values of all the compounds may be between four- and five-fold higher in the SYBR Green assay compared to the 3H-hypoxanthine-based assay, which measures incorporation of tritium into DNA, as the optimal fluorescent reading conditions required the use of albumax, a lipid-enriched bovine albumin to which small molecules bind. This binding effectively sequesters the drug, thereby reducing the compound concentration and shifting the EC50 to a higher value. A shift in the EC50 due to albumax was also observed for the reference inhibitors chloroquine and artemisinin, which yielded EC50 values of less than 10 nM with the 3D7 strain in the 3H-hypoxanthine-based assay under low protein condition but showed an EC50 in the same range as the best compound in the parasite proliferation assay (70 nM).