 2,5'-dichloro-5'-deoxy-N6-cyclopentyladenosine BDBM50267394 CHEMBL477445
2,5'-dichloro-5'-deoxy-N6-cyclopentyladenosine BDBM50267394 CHEMBL477445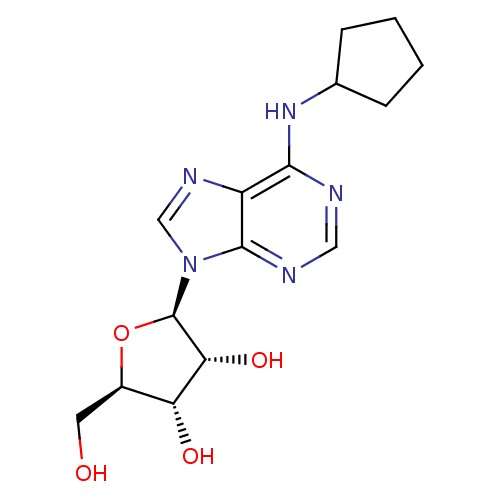 N6-cyclopentyladenosine (CPA) cid_657378 CPA N6-CyclopentylAdo BDBM25400 CHEMBL68738 (2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol
N6-cyclopentyladenosine (CPA) cid_657378 CPA N6-CyclopentylAdo BDBM25400 CHEMBL68738 (2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol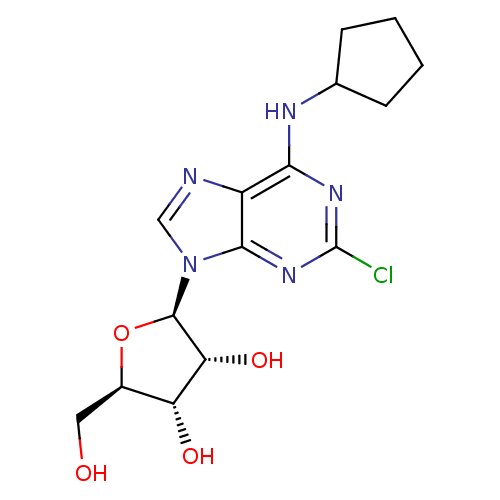 (2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol N6-Cyclopentyl-2-chloroAdo 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol(CCPA) 2-Cl-CPA BDBM50085658 2-chloro-N6-cyclopentyladenosine (2R,3R,4S,5R)-2-(2-chloro-6-(cyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)-tetrahydrofuran-3,4-diol 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol CHEMBL284969 CCPA 5-(2-Chloro-6-cyclopentylamino-purin-9-yl)-tetrahydro-furan-2,3,4-triol
(2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol N6-Cyclopentyl-2-chloroAdo 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol(CCPA) 2-Cl-CPA BDBM50085658 2-chloro-N6-cyclopentyladenosine (2R,3R,4S,5R)-2-(2-chloro-6-(cyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)-tetrahydrofuran-3,4-diol 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol CHEMBL284969 CCPA 5-(2-Chloro-6-cyclopentylamino-purin-9-yl)-tetrahydro-furan-2,3,4-triol
- ChEMBL_859652 (CHEMBL2168889) Displacement of [3H]2-chloro-N6-cyclopentyladenosine from human A3AR
- ChEMBL_859653 (CHEMBL2168890) Displacement of [3H]2-chloro-N6-cyclopentyladenosine from human A2AR
- ChEMBL_859785 (CHEMBL2169613) Displacement of [3H]2-chloro-N6-cyclopentyladenosine from human A1AR
- ChEMBL_1707355 (CHEMBL4058588) Displacement of [3H]-NECA from A2A receptor in rat striatal membrane in presence of A1 receptor agonist N6-cyclopentyladenosine
- ChEMBL_30695 (CHEMBL644770) Inhibition of [3H]5'-(N-ethylcarbamoyl)-adenosine binding to adenosine A2 receptor in rat striatal membranes with 50 nM cyclopentyladenosine
- ChEMBL_31029 (CHEMBL638318) Binding affinity for Adenosine A2 receptor using N-[3H]-ethyladenosine-5`-uronamide in presence of cyclopentyladenosine rat striatal membranes under usual light
- ChEMBL_2224406 (CHEMBL5137919) Displacement of [3H]NECA from A2AAR in rat striata assessed as inhibition constant in presence of N6-cyclopentyladenosine by radioligand competitive binding assay
- ChEMBL_30694 (CHEMBL644769) Inhibition of [3H]5'-(N-ethylcarbamoyl)-adenosine binding to Adenosine A2 receptor in rat striatal membranes in the presence of 50 nM cyclopentyladenosine
- ChEMBL_30716 (CHEMBL646483) Binding affinity against adenosine A2 receptor in rat striatal membranes using N-[3H]-ethyladenosin-5''-uronamide as radioligand in the presence of 50 nM cyclopentyladenosine
- ChEMBL_31035 (CHEMBL638323) Binding affinity carried out with [3H]5'-(N-ethylcarbamoyl)-adenosine in the presence of 50 nM cyclopentyladenosine in rat striatal membranes against adenosine A2 receptor.
- Binding Assay For binding to the human A1AR, [3H]R-PIA (2 nM) was incubated with membranes (40 μg/tube) from CHO cells stably expressing the human A1AR at 25° C. for 60 min in 50 mM Tris.HCl buffer (pH 7.4; MgCl2, 10 mM) in a total assay volume of 200 μL. Nonspecific binding was determined using 10 μM of N6-cyclopentyladenosine. For human A2A AR binding, membranes (20 μg/tube) from HEK-293 cells stably expressing the human A2A AR were incubated with 15 nM [3H]CGS21680 at 25° C. for 60 min in 200 μL of 50 mM Tris.HCl, pH 7.4, containing 10 mM MgCl2. N-5′-ethyluronamidoadenosine (10 μM) was used to define nonspecific binding. Reaction was terminated by filtration with GF/B filters.
- Radioligand Binding Assay Membranes for radioligand binding experiments are prepared from fresh or frozen cells as described in Klotz et al., Naunyn-Schmiedeberg's Arch. Pharmacol, 357:1-9 (1998). The cell suspension is then homogenized in ice-cold hypotonic buffer (5 mM Tris/HCl, 2 mM EDTA, pH 7.4) and the homogenate is spun for 10 minutes (4° C.) at 1,000 g. The membranes are then sedimented from the supernatant for 30 minutes at 100,000 g and resuspended in 50 mM Tris/HCl buffer pH 7.4 (for A3 adenosine receptors: 50 mM Tris/HCl, 10 mM MgCl2, 1 mM EDTA, pH 8.25), frozen in liquid nitrogen at a protein concentration of 1-3 mg/mL and stored at −80° C. Dissociation constants of unlabeled compounds (Ki-values) are determined in competition experiments in 96-well microplates using the A1 selective agonist 2-chloro-N6-[3H]cyclopentyladenosine ([3H]CCPA, 1 nM) for the characterization of A1 receptor binding. Nonspecific binding is determined in the presence of 100 M R-PM and 1 mM theophylline, respectively.
- Adenosine A1 receptor CHO-K1/A1R cells were cultured in DMEM/F12 medium containing 10% fetal bovine serum and 1 mg/ml G418. The cells were digested with the cell separation buffer during the experiment. The cells were then resuspended in the balanced salt buffer containing 20 mM HEPES and 0.1% bovine serum albumin and counted, and the cell density was adjusted to 5×105 cells/ml. In the 384-well plate, each well was added with 12.5 μl of cell suspension, and 6.25 μl of test compound (4× concentration) formulated with the balanced salt buffer containing 20 mM HEPES, 0.1% bovine serum albumin, 54 μM rolipram and 2.7 U/ml adenosine deaminase, and the plate was incubated at room temperature for 30 minutes. Each well was then added with 6.25 μl of forskolin and N6-cyclopentyladenosine (4× concentration) formulated with the balanced salt buffer containing 20 mM HEPES, 0.1% bovine serum albumin, 54 μM rolipram and 2.7 U/ml adenosine deaminase, and the plate was incubated at room temperature for 30 minutes. The final concentrations of the compounds were: 100000, 10000, 1000, 100, 10, 1, 0.1 and 0 nM. The final concentration of forskolin was 10 μM. The final concentration of CPA was 10 nM. Intracellular cAMP concentration was detected with the cAMP dynamic 2 kit. cAMP-d2 and Anti-cAMP-Eu-Cryptate were diluted respectively with the cAMP lysis buffer at a ratio of 1:4. Each well was added with 12.5 μl of diluted cAMP-d2, followed by addition of 12.5 μl of diluted Anti-cAMP-Eu-Cryptate, and the plate was incubated at room temperature in the dark for 1 hour. The HTRF signal values were read by the PHERAstar multi-function microplate reader. IC50 values of inhibition activity of the compounds were calculated by Graphpad Prism software.
- Binding Assays [3H]R N6-Phenylisopropyladenosine (40, [3H]R-PIA, 63 Ci/mmol), [3H](2-[p-(2-carboxyethyl)phenyl-ethylamino]-5′-N-ethylcarboxamido-adenosine) (41, [3H]CGS21680, 40.5 Ci/mmol) and [25I]N6-(4-amino-3-iodobenzyl)adenosine-5′-N-methyluronamide (42, [125I]I-AB-MECA, 2200 Ci/mmol) were purchased from Perkin-Elmer Life and Analytical Science (Boston, Mass.). Test compounds were prepared as 5 mM stock solutions in DMSO and stored frozen. Pharmacological standards 1b (A3AR agonist), adenosine-5′-N-ethylcarboxamide (43, NECA, nonselective AR agonist) and 2-chloro-N6-cyclopentyladenosine (44, CCPA, A1AR agonist) were purchased from Tocris R&D Systems (Minneapolis, Minn.).Cell Culture and Membrane Preparation CHO cells stably expressing the recombinant hA1 and hA3ARs and HEK293 cells stably expressing the hA2AAR were cultured in Dulbecco's modified Eagle medium (DMEM) and F12 (1:1) supplemented with 10% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 μmol/mL glutamine. In addition, 800 μg/mL geneticin was added to the A2A media, while 500 μg/mL hygromycin was added to the A1 and A3 media. After harvesting, cells were homogenized and suspended in PBS. Cells were then centrifuged at 240 g for 5 min, and the pellet was resuspended in 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2. The suspension was homogenized and was then ultra-centrifuged at 14,330 g for 30 min at 4° C. The resultant pellets were resuspended in Tris buffer, incubated with adenosine deaminase (3 units/mL) for 30 min at 37° C. The suspension was homogenized with an electric homogenizer for 10 sec, pipetted into 1 mL vials and then stored at −80° C. until the binding experiments. The protein concentration was measured using the BCA Protein Assay Kit from Pierce Biotechnology, Inc. (Rockford, Ill.).27Binding Assays:Into each tube in the binding assay was added 50 μL of increasing concentrations of the test ligand in Tris-HCl buffer (50 mM, pH 7.5) containing 10 mM MgCl2, 50 μL of the appropriate agonist radioligand, and finally 100 μL of membrane suspension. For the A1AR (22 μg of protein/tube) the radioligand used was [3H]40 (final concentration of 3.5 nM). For the A2AAR (20 μg/tube) the radioligand used was [3H]41 (10 nM). For the A3AR (21 μg/tube) the radioligand used was [125I]42 (0.34 nM). Nonspecific binding was determined using a final concentration of 10 μM 43 diluted with the buffer. The mixtures were incubated at 25° C. for 60 min in a shaking water bath. Binding reactions were terminated by filtration through Brandel GF/B filters under a reduced pressure using a M-24 cell harvester (Brandel, Gaithersburg, Md.). Filters were washed three times with 3 mL of 50 mM ice-cold Tris-HCl buffer (pH 7.5). Filters for A1 and A2AAR binding were placed in scintillation vials containing 5 mL of Hydrofluor scintillation buffer and counted using a Perkin Elmer Liquid Scintillation Analyzer (Tri-Carb 2810TR). Filters for A3AR binding were counted using a Packard Cobra II γ-counter. The Ki values were determined using GraphPad Prism for all assays.Similar competition binding assays were conducted using HEK293 cell membranes expressing mARs using [125I]42 to label A1 or A3ARs and [3H]41 to label A2AARs. IC50 values were converted to Ki values as described.28 Nonspecific binding was determined in the presence of 100 μM 43.
 2,5'-dichloro-5'-deoxy-N6-cyclopentyladenosine BDBM50267394 CHEMBL477445
2,5'-dichloro-5'-deoxy-N6-cyclopentyladenosine BDBM50267394 CHEMBL477445 N6-cyclopentyladenosine (CPA) cid_657378 CPA N6-CyclopentylAdo BDBM25400 CHEMBL68738 (2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol
N6-cyclopentyladenosine (CPA) cid_657378 CPA N6-CyclopentylAdo BDBM25400 CHEMBL68738 (2R,3R,4S,5R)-2-[6-(cyclopentylamino)-9H-purin-9-yl]-5-(hydroxymethyl)oxolane-3,4-diol (2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol N6-Cyclopentyl-2-chloroAdo 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol(CCPA) 2-Cl-CPA BDBM50085658 2-chloro-N6-cyclopentyladenosine (2R,3R,4S,5R)-2-(2-chloro-6-(cyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)-tetrahydrofuran-3,4-diol 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol CHEMBL284969 CCPA 5-(2-Chloro-6-cyclopentylamino-purin-9-yl)-tetrahydro-furan-2,3,4-triol
(2R,3R,4S,5R)-2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol N6-Cyclopentyl-2-chloroAdo 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol(CCPA) 2-Cl-CPA BDBM50085658 2-chloro-N6-cyclopentyladenosine (2R,3R,4S,5R)-2-(2-chloro-6-(cyclopentylamino)-9H-purin-9-yl)-5-(hydroxymethyl)-tetrahydrofuran-3,4-diol 2-(2-Chloro-6-cyclopentylamino-purin-9-yl)-5-hydroxymethyl-tetrahydro-furan-3,4-diol CHEMBL284969 CCPA 5-(2-Chloro-6-cyclopentylamino-purin-9-yl)-tetrahydro-furan-2,3,4-triol