Query String: DCA
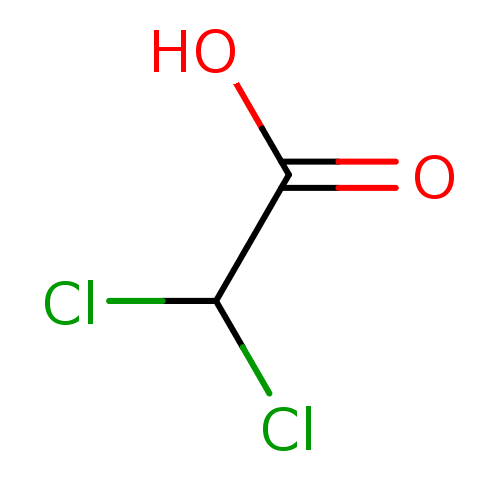 DCA BDBM227588
DCA BDBM227588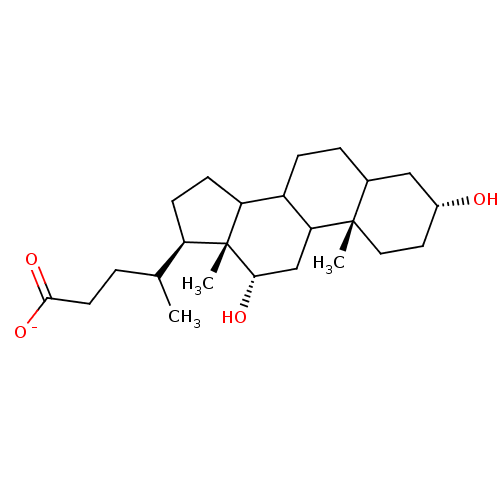 deoxycholate (DCA) BDBM36168
deoxycholate (DCA) BDBM36168 lago-DCA CHEMBL1609669 BDBM50423621
lago-DCA CHEMBL1609669 BDBM50423621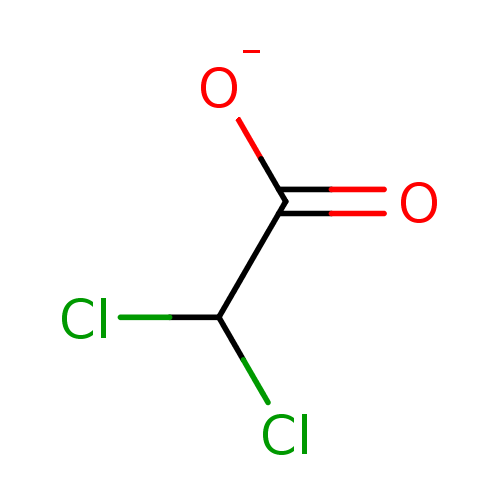 CPC-211 Ceresine DCA BDBM50177027 Sodium dichloroacetate
CPC-211 Ceresine DCA BDBM50177027 Sodium dichloroacetate BDBM23739 DMPC-DCA Phospholipid Blend dimyristoylphosphatidylcholine-deoxycholic acid
BDBM23739 DMPC-DCA Phospholipid Blend dimyristoylphosphatidylcholine-deoxycholic acid
- Biochemical Activity Assay The PDC inactivation assay is performed in Greiner 384-well microtiter plates and is used for high throughput screen. 4 μl of PDHK2 (human, rec, Carna Bioscience, 10 ng/μl-137 nM final concentration) and PDC (isolated from porcine heart, Sigma-Aldrich, 20 mU/ml final concentration) are incubated in the absence or presence of the test compound (10 dilution concentrations) for 30 min at room temperature in kinase buffer (15 mM potassium phosphate buffer, pH 7.0, 60 mM KCl, 1.5 mM DTT, 2.5 mM MgCl2, 0.0125% (w/v) BSA, 0.125% Pluronic F-68). The kinase reaction is started by the addition of 4 l ATP substrate solution (fc 5 μM in kinase buffer). After 30 min incubation at 37° C. 40 μl of PDC reaction solution (100 mM Tris/HCl, pH 7.8, 0.5 mM EDTA, 1 mM MgCl2, 50 mM NaF, 0.25 mM Coenzyme A, 5 mM pyruvate, 1 mM NAD, 5 mM DTT, 1 mM thiamine pyrophosphate) is added. The first fluorescence measurement is performed on a Perkin Elmer Envision (Exc 340 nm, Em 450 nm). The reaction is incubated for 45 min at room temperature. Afterwards a second fluorescence measurement is performed and the PDC activity is calculated by the difference between both measurements. As full value for the PDHK2 assay the inhibitor-free PDHK2 reaction is used. The pharmacological zero value used is DCA (Sigma-Aldrich) in a final concentration of 3 mM. The inhibitory values (IC50) were determined using either the program Symyx Assay Explorer or Condosseo from GeneData.
- Biochemical Activity Testing of PDHK2 The biochemical activity assay for PDHK2 is based on the inactivation of PDC through phosphorylation by PDHK2. The assay is run in two steps: the enzymatic PDHK2 reaction in which isolated PDC is phosphorylated by PDHK2 with ATP as co-substrate and the PDC activity assay in which pyruvate and NAD are converted to acetyl-CoA and NADH. The PDC activity correlates to the increase in NADH and thereby is detectable directly via the increasing fluorescence signal (Exc 340 nm, Em 450 nm). Inhibition of PDHK2 results in a lower phosphorylation status and thereby a less decrease in activity of PDC and a stronger increase in NADH fluorescence signal.The PDC inactivation assay is performed in Greiner 384-well microtiter plates and is used for high throughput screen. 4 μl of PDHK2 (human, rec, Carna Bioscience, 10 ng/μl-137 nM final concentration) and PDC (isolated from porcine heart, Sigma-Aldrich, 20 mU/ml final concentration) are incubated in the absence or presence of the test compound (10 dilution concentrations) for 30 min at room temperature in kinase buffer (15 mM potassium phosphate buffer, pH 7.0, 60 mM KCl, 1.5 mM DTT, 2.5 mM MgCl2, 0.0125% (w/v) BSA, 0.125% Pluronic F-68). The kinase reaction is started by the addition of 4 μl ATP substrate solution (fc 5 μM in kinase buffer). After 30 min incubation at 37° C. 40 μl of PDC reaction solution (100 mM Tris/HCl, pH 7.8, 0.5 mM EDTA, 1 mM MgCl2, 50 mM NaF, 0.25 mM Coenzyme A, 5 mM pyruvate, 1 mM NAD, 5 mM DTT, 1 mM thiamine pyrophosphate) is added. The first fluorescence measurement is performed on a Perkin Elmer Envision (Exc 340 nm, Em 450 nm). The reaction is incubated for 45 min at room temperature. Afterwards a second fluorescence measurement is performed and the PDC activity is calculated by the difference between both measurements. As full value for the PDHK2 assay the inhibitor-free PDHK2 reaction is used. The pharmacological zero value used is DCA (Sigma-Aldrich) in a final concentration of 3 mM. The inhibitory values (1050) were determined using either the program Symyx Assay Explorer or Condosseo from GeneData.




