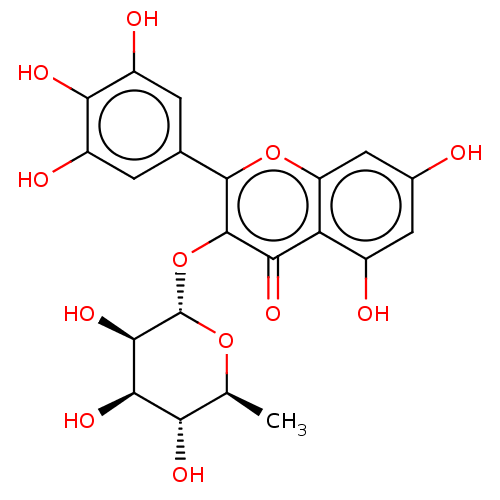
 5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one BDBM153266 Myricitrin (7)
5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one BDBM153266 Myricitrin (7) 3-[6-methyl-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5,7-bis(oxidanyl)-2-[3,4,5-tris(oxidanyl)phenyl]chromen-4-one BDBM115130 MYRICITRIN MLS000737865 SMR000528196 5,7-dihydroxy-3-[(3,4,5-trihydroxy-6-methyl-2-oxanyl)oxy]-2-(3,4,5-trihydroxyphenyl)-1-benzopyran-4-one cid_5352000 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromone 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one
3-[6-methyl-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5,7-bis(oxidanyl)-2-[3,4,5-tris(oxidanyl)phenyl]chromen-4-one BDBM115130 MYRICITRIN MLS000737865 SMR000528196 5,7-dihydroxy-3-[(3,4,5-trihydroxy-6-methyl-2-oxanyl)oxy]-2-(3,4,5-trihydroxyphenyl)-1-benzopyran-4-one cid_5352000 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromone 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one
- Rakers, C; Schwerdtfeger, SM; Mortier, J; Duwe, S; Wolff, T; Wolber, G; Melzig, MF Inhibitory potency of flavonoid derivatives on influenza virus neuraminidase. Bioorg Med Chem Lett 24: 4312-7 (2014)
- Hu, X; Wu, JW; Wang, M; Yu, MH; Zhao, QS; Wang, HY; Hou, AJ 2-Arylbenzofuran, flavonoid, and tyrosinase inhibitory constituents of Morus yunnanensis. J Nat Prod 75: 82-7 (2012)
- Obreque-Balboa, JE; Sun, Q; Bernhardt, G; König, B; Buschauer, A Flavonoid derivatives as selective ABCC1 modulators: Synthesis and functional characterization. Eur J Med Chem 109: 124-33 (2016)
- Cushman, M; Nagarathnam, D; Burg, DL; Geahlen, RL Synthesis and protein-tyrosine kinase inhibitory activities of flavonoid analogues. J Med Chem 34: 798-806 (1991)
- Sheng, R; Lin, X; Zhang, J; Chol, KS; Huang, W; Yang, B; He, Q; Hu, Y Design, synthesis and evaluation of flavonoid derivatives as potent AChE inhibitors. Bioorg Med Chem 17: 6692-8 (2009)
- Shrestha, S; Natarajan, S; Park, JH; Lee, DY; Cho, JG; Kim, GS; Jeon, YJ; Yeon, SW; Yang, DC; Baek, NI Potential neuroprotective flavonoid-based inhibitors of CDK5/p25 from Rhus parviflora. Bioorg Med Chem Lett 23: 5150-4 (2013)
- Li, K; Diakite, D; Austin, J; Lee, JH; Lantvit, DD; Murphy, BT; Burdette, JE The Flavonoid Baicalein Negatively Regulates Progesterone Target Genes in the Uterus J Nat Prod 85: 237-247 (2022)
- Holder, S; Lilly, M; Brown, ML Comparative molecular field analysis of flavonoid inhibitors of the PIM-1 kinase. Bioorg Med Chem 15: 6463-73 (2007)
- Karton, Y; Jiang, JL; Ji, XD; Melman, N; Olah, ME; Stiles, GL; Jacobson, KA Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem 39: 2293-301 (1996)
- McArdle, BM; Campitelli, MR; Quinn, RJ A common protein fold topology shared by flavonoid biosynthetic enzymes and therapeutic targets. J Nat Prod 69: 14-7 (2006)
- Cao, H; Pauff, JM; Hille, R X-ray crystal structure of a xanthine oxidase complex with the flavonoid inhibitor quercetin. J Nat Prod 77: 1693-9 (2014)
- Jalili-Baleh, L; Babaei, E; Abdpour, S; Nasir Abbas Bukhari, S; Foroumadi, A; Ramazani, A; Sharifzadeh, M; Abdollahi, M; Khoobi, M A review on flavonoid-based scaffolds as multi-target-directed ligands (MTDLs) for Alzheimer's disease. Eur J Med Chem 152: 570-589 (2018)
- Park, KH; Eom, SH; Ryu, YB; Cho, JK Composition for suppressing neuraminidase activity comprising geranylated flavonoid derived from Paulownia tomentosa as active ingredient US Patent US10406136 (2019)
- Luo, W; Wang, T; Hong, C; Yang, YC; Chen, Y; Cen, J; Xie, SQ; Wang, CJ Design, synthesis and evaluation of 4-dimethylamine flavonoid derivatives as potential multifunctional anti-Alzheimer agents. Eur J Med Chem 122: 17-26 (2016)
- Li, RS; Wang, XB; Hu, XJ; Kong, LY Design, synthesis and evaluation of flavonoid derivatives as potential multifunctional acetylcholinesterase inhibitors against Alzheimer's disease. Bioorg Med Chem Lett 23: 2636-41 (2013)
- Tsuchiya, A; Kobayashi, M; Kamatari, YO; Mitsunaga, T; Yamauchi, K Development of flavonoid probes and the binding mode of the target protein and quercetin derivatives. Bioorg Med Chem 68: (2022)
- Discovery of novel flavonoid-based CDK9 degraders for prostate cancer treatment via a PROTAC strategy.
- Wong, ILK; Zhu, X; Chan, KF; Liu, Z; Chan, CF; Chow, TS; Chong, TC; Law, MC; Cui, J; Chow, LMC; Chan, TH Flavonoid Monomers as Potent, Nontoxic, and Selective Modulators of the Breast Cancer Resistance Protein (ABCG2). J Med Chem 64: 14311-14331 (2021)
- Siah, M; Farzaei, MH; Ashrafi-Kooshk, MR; Adibi, H; Arab, SS; Rashidi, MR; Khodarahmi, R Inhibition of guinea pig aldehyde oxidase activity by different flavonoid compounds: An in vitro study. Bioorg Chem 64: 74-84 (2016)
- Zhang, S; Ma, J; Bao, Y; Yang, P; Zou, L; Li, K; Sun, X Nitrogen-containing flavonoid analogues as CDK1/cyclin B inhibitors: synthesis, SAR analysis, and biological activity. Bioorg Med Chem 16: 7128-33 (2008)
- Rho, HS; Ahn, SM; Lee, BC; Kim, MK; Ghimeray, AK; Jin, CW; Cho, DH Changes in flavonoid content and tyrosinase inhibitory activity in kenaf leaf extract after far-infrared treatment. Bioorg Med Chem Lett 20: 7534-6 (2010)
- Ma, ML; Li, M; Gou, JJ; Ruan, TY; Jin, HS; Zhang, LH; Wu, LC; Li, XY; Hu, YH; Wen, K; Zhao, Z Design, synthesis and biological activity of flavonoid derivatives as selective agonists for neuromedin U 2 receptor. Bioorg Med Chem 22: 6117-23 (2014)
- Luo, W; Chen, Y; Wang, T; Hong, C; Chang, LP; Chang, CC; Yang, YC; Xie, SQ; Wang, CJ Design, synthesis and evaluation of novel 7-aminoalkyl-substituted flavonoid derivatives with improved cholinesterase inhibitory activities. Bioorg Med Chem 24: 672-80 (2016)
- Kusakabe, Y; Moriya, SS; Sugiyama, T; Miyata, Y Isolation and identification of the new baicalin target protein to develop flavonoid structure-based therapeutic agents. Bioorg Med Chem 90: (2023)
- Kishore, N; Twilley, D; Blom van Staden, A; Verma, P; Singh, B; Cardinali, G; Kovacs, D; Picardo, M; Kumar, V; Lall, N Isolation of Flavonoids and Flavonoid Glycosides from Myrsine africana and Their Inhibitory Activities against Mushroom Tyrosinase. J Nat Prod 81: 49-56 (2018)
- Sugimoto, S; Yamano, Y; Desoukey, SY; Katakawa, K; Wanas, AS; Otsuka, H; Matsunami, K Isolation of Sesquiterpene-Amino Acid Conjugates, Onopornoids A-D, and a Flavonoid Glucoside from Onopordum alexandrinum. J Nat Prod 82: 1471-1477 (2019)
- Campana, PR; Coleman, CM; Sousa, LP; Teixeira, MM; Ferreira, D; Braga, FC Mansoins C-F, Oligomeric Flavonoid Glucosides Isolated from Mansoa hirsuta Fruits with Potential Anti-inflammatory Activity. J Nat Prod 79: 2279-86 (2016)
- Lu, W; Zhu, J; Zou, S; Li, X; Huang, J The efficient expression of human fibroblast collagenase in Escherichia coli and the discovery of flavonoid inhibitors. J Enzyme Inhib Med Chem 28: 741-6 (2013)
- Huh, J; Ha, TKQ; Kang, KB; Kim, KH; Oh, WK; Kim, J; Sung, SH C-Methylated Flavonoid Glycosides from Pentarhizidium orientale Rhizomes and Their Inhibitory Effects on the H1N1 Influenza Virus. J Nat Prod 80: 2818-2824 (2017)
- Luo, W; Su, YB; Hong, C; Tian, RG; Su, LP; Wang, YQ; Li, Y; Yue, JJ; Wang, CJ Design, synthesis and evaluation of novel 4-dimethylamine flavonoid derivatives as potential multi-functional anti-Alzheimer agents. Bioorg Med Chem 21: 7275-82 (2013)
- Nguyen, DH; Seo, UM; Zhao, BT; Le, DD; Seong, SH; Choi, JS; Min, BS; Woo, MH Ellagitannin and flavonoid constituents from Agrimonia pilosa Ledeb. with their protein tyrosine phosphatase and acetylcholinesterase inhibitory activities Bioorg Chem 72: 293-300 (2017)
- Parellada, J; Guinea, M Flavonoid Inhibitors of Trypsin and Leucine Aminopeptidase: A Proposed Mathematical Model for IC50 Estimation J Nat Prod 58: 823-829 (1995)
- Thao, NP; Luyen, BT; Kim, JH; Jo, AR; Dat, NT; Kiem, PV; Minh, CV; Kim, YH Identification, characterization, kinetics, and molecular docking of flavonoid constituents from Archidendron clypearia (Jack.) Nielsen leaves and twigs. Bioorg Med Chem 24: 3125-32 (2016)
- Yen, SC; Chen, LC; Huang, HL; Ngo, ST; Wu, YW; Lin, TE; Sung, TY; Lien, ST; Tseng, HJ; Pan, SL; Huang, WJ; Hsu, KC Investigation of Selected Flavonoid Derivatives as Potent FLT3 Inhibitors for the Potential Treatment of Acute Myeloid Leukemia. J Nat Prod 84: 1-10 (2021)
- Zhu, X; Wong, ILK; Chan, KF; Cui, J; Law, MC; Chong, TC; Hu, X; Chow, LMC; Chan, TH Triazole Bridged Flavonoid Dimers as Potent, Nontoxic, and Highly Selective Breast Cancer Resistance Protein (BCRP/ABCG2) Inhibitors. J Med Chem 62: 8578-8608 (2019)
- Su, ZR; Fan, SY; Shi, WG; Zhong, BH Discovery of xanthine oxidase inhibitors and/ora-glucosidase inhibitors by carboxyalkyl derivatization based on the flavonoid of apigenin. Bioorg Med Chem Lett 25: 2778-81 (2015)
- Estrada Valencia, M; Herrera-Arozamena, C; de Andrés, L; Pérez, C; Morales-García, JA; Pérez-Castillo, A; Ramos, E; Romero, A; Viña, D; Yáñez, M; Laurini, E; Pricl, S; Rodríguez-Franco, MI Neurogenic and neuroprotective donepezil-flavonoid hybrids with sigma-1 affinity and inhibition of key enzymes in Alzheimer's disease. Eur J Med Chem 156: 534-553 (2018)
- Chan, KF; Wong, IL; Kan, JW; Yan, CS; Chow, LM; Chan, TH Amine linked flavonoid dimers as modulators for P-glycoprotein-based multidrug resistance: structure-activity relationship and mechanism of modulation. J Med Chem 55: 1999-2014 (2012)
- Design and optimization of selective and potent CDK9 inhibitors with flavonoid scaffold for the treatment of acute myeloid leukemia.
- Li, SY; Wang, XB; Xie, SS; Jiang, N; Wang, KD; Yao, HQ; Sun, HB; Kong, LY Multifunctional tacrine-flavonoid hybrids with cholinergic,ß-amyloid-reducing, and metal chelating properties for the treatment of Alzheimer's disease. Eur J Med Chem 69: 632-46 (2013)
- Li, BW; Zhang, FH; Serrao, E; Chen, H; Sanchez, TW; Yang, LM; Neamati, N; Zheng, YT; Wang, H; Long, YQ Design and discovery of flavonoid-based HIV-1 integrase inhibitors targeting both the active site and the interaction with LEDGF/p75. Bioorg Med Chem 22: 3146-58 (2014)
- Tang, G; Ding, K; Nikolovska-Coleska, Z; Yang, CY; Qiu, S; Shangary, S; Wang, R; Guo, J; Gao, W; Meagher, J; Stuckey, J; Krajewski, K; Jiang, S; Roller, PP; Wang, S Structure-based design of flavonoid compounds as a new class of small-molecule inhibitors of the anti-apoptotic Bcl-2 proteins. J Med Chem 50: 3163-6 (2007)
- Moro, S; van Rhee, AM; Sanders, LH; Jacobson, KA Flavonoid derivatives as adenosine receptor antagonists: a comparison of the hypothetical receptor binding site based on a comparative molecular field analysis model. J Med Chem 41: 46-52 (1998)
- Liu, Z; Wong, ILK; Sang, J; Liu, F; Yan, CSW; Kan, JWY; Chan, TH; Chow, LMC Identification of Binding Sites in the Nucleotide-Binding Domain of P-Glycoprotein for a Potent and Nontoxic Modulator, the Amine-Containing Monomeric Flavonoid J Med Chem 66: 6160-6183 (2023)
- Jia, Y; Hoang, MH; Jun, HJ; Lee, JH; Lee, SJ Cyanidin, a natural flavonoid, is an agonistic ligand for liver X receptor alpha and beta and reduces cellular lipid accumulation in macrophages and hepatocytes. Bioorg Med Chem Lett 23: 4185-90 (2013)
- Wong, ILK; Zhu, X; Chan, KF; Law, MC; Lo, AMY; Hu, X; Chow, LMC; Chan, TH Discovery of Novel Flavonoid Dimers To Reverse Multidrug Resistance Protein 1 (MRP1, ABCC1) Mediated Drug Resistance in Cancers Using a High Throughput Platform with "Click Chemistry". J Med Chem 61: 9931-9951 (2018)
- DPP III Enzyme Activity Assay The inhibitory activity of flavonoids toward human DPP III was assayed in a 50 mM Tris-HCl buffer, pH 7.4. In brief, recombinant human DPP III (0.29 nM) was preincubated with increasing concentrations of flavonoid first for 1 min at 25°C and then for 3 min at 37°C. The enzymatic reaction was started with Arg-Arg-NA (40 μM) as a substrate, and after the 15 min incubation at 37°C in a water bath, the reaction was stopped and the absorbance was measured using the spectrophotometric method described above[Abramić et al., Biol. Chem. Hoppe-Seyler., 369:29]. The stock solutions of flavonoids were prepared daily in dimethyl sulfoxide (DMSO, Sigma-Aldrich).
- Thioflavin-T (Th-T) Fluorescence Assay Fluorescence intensity was measured at 420 nm excitation and 485 nm emission using a microplate reader (MPR-A4 II; TOSOH, Tokyo, Japan, or Fluoroskan Ascent; Thermo Scientific, Rockford, IL). In brief, Aβ42 was dissolved in 0.1% NH4OH at 250 uM, and each flavonoid was dissolved in EtOH at 5 mM, followed by dilution with sodiumphosphate-buffered saline (PBS: 50 mM sodium phosphate and 100 mM NaCl, pH 7.4) at the desired concentration (Aβ42, 25 uM; flavonoids, 50 uM). NaIO4 or Tris(2-carboxyethyl)phosphine hydrochloride (TCEP-HCl) was initially dissolved in PBSat 100 mM, then diluted with PBS at 100 uM before use. Experiments under an anaerobic condition were performed in a desiccator evacuated by a diaphragm pump (about 8mmHg; KNF Lab LABOPORT vacuum pump, KNF Neuberger, NJ) at room temperature. Unless otherwise noted, the concentrations of Aβ42, flavonoids, and oxidant/reductant used in this study were 25, 50, and 100 uM, respectively.
 5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one BDBM153266 Myricitrin (7)
5,7-dihydroxy-3-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one BDBM153266 Myricitrin (7) 3-[6-methyl-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5,7-bis(oxidanyl)-2-[3,4,5-tris(oxidanyl)phenyl]chromen-4-one BDBM115130 MYRICITRIN MLS000737865 SMR000528196 5,7-dihydroxy-3-[(3,4,5-trihydroxy-6-methyl-2-oxanyl)oxy]-2-(3,4,5-trihydroxyphenyl)-1-benzopyran-4-one cid_5352000 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromone 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one
3-[6-methyl-3,4,5-tris(oxidanyl)oxan-2-yl]oxy-5,7-bis(oxidanyl)-2-[3,4,5-tris(oxidanyl)phenyl]chromen-4-one BDBM115130 MYRICITRIN MLS000737865 SMR000528196 5,7-dihydroxy-3-[(3,4,5-trihydroxy-6-methyl-2-oxanyl)oxy]-2-(3,4,5-trihydroxyphenyl)-1-benzopyran-4-one cid_5352000 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyl-tetrahydropyran-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromone 5,7-dihydroxy-3-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one