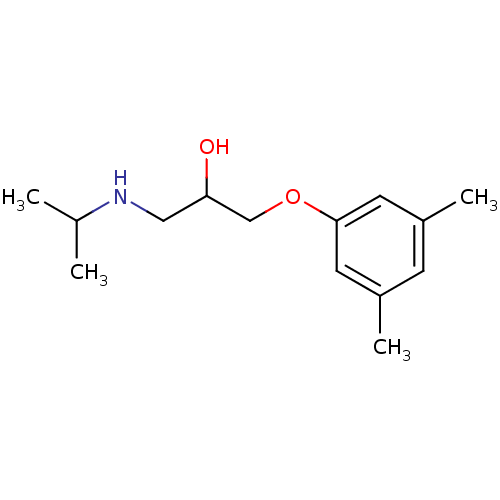
 CHEMBL1159714 Ko 707 BDBM50421719
CHEMBL1159714 Ko 707 BDBM50421719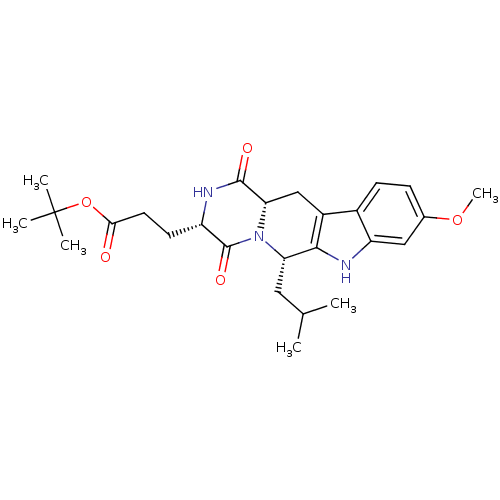 3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester BDBM50305083 3-((3S,6S,12aS)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester Ko143 Ko-143 US9695174, Ko143 CHEMBL488910
3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester BDBM50305083 3-((3S,6S,12aS)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester Ko143 Ko-143 US9695174, Ko143 CHEMBL488910
- Miyano, N; Kinoshita, T; Nakai, R; Kirii, Y; Yokota, K; Tada, T Structural basis for the inhibitor recognition of human Lyn kinase domain. Bioorg Med Chem Lett 19: 6557-60 (2009)
- Horio, T; Hamasaki, T; Inoue, T; Wakayama, T; Itou, S; Naito, H; Asaki, T; Hayase, H; Niwa, T Structural factors contributing to the Abl/Lyn dual inhibitory activity of 3-substituted benzamide derivatives. Bioorg Med Chem Lett 17: 2712-7 (2007)
- Dolan, BM; Duron, SG; Campbell, DA; Vollrath, B; Shankaranarayana Rao, BS; Ko, HY; Lin, GG; Govindarajan, A; Choi, SY; Tonegawa, S Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc Natl Acad Sci U S A 110: 5671-6
- Kohal, R; Bhavana, na; Kumari, P; Sharma, AK; Gupta, GD; Verma, SK Fyn, Blk, and Lyn kinase inhibitors: A mini-review on medicinal attributes, research progress, and future insights. Bioorg Med Chem Lett 102:
- Fallacara, AL; Passannanti, R; Mori, M; Iovenitti, G; Musumeci, F; Greco, C; Crespan, E; Kissova, M; Maga, G; Tarantelli, C; Spriano, F; Gaudio, E; Bertoni, F; Botta, M; Schenone, S Identification of a new family of pyrazolo[3,4-d]pyrimidine derivatives as multitarget Fyn-Blk-Lyn inhibitors active on B- and T-lymphoma cell lines. Eur J Med Chem 181: (2019)
- Lipinski, CA; Reaume, AG High throughput in vivo phenotypic screening for drug repurposing: Discovery of MLR-1023 a novel insulin sensitizer and novel Lyn kinase activator with clinical proof of concept. Bioorg Med Chem 28: (2020)
- Elliott, RL; Cameron, KO; Chin, JE; Bartlett, JA; Beretta, EE; Chen, Y; Jardine, Pda S; Dubins, JS; Gillaspy, ML; Hargrove, DM; Kalgutkar, AS; LaFlamme, JA; Lame, ME; Martin, KA; Maurer, TS; Nardone, NA; Oliver, RM; Scott, DO; Sun, D; Swick, AG; Trebino, CE; Zhang, Y Bioorg Med Chem Lett 20: 6797-801 (2010)
- Pokhrel, L; Maezawa, I; Nguyen, TD; Chang, KO; Jin, LW; Hua, DH J Med Chem 55: 8969-73 (2012)
- Clausen, JD; Kjellerup, L; Cohrt, KO; Hansen, JB; Dalby-Brown, W; Winther, AL Bioorg Med Chem Lett 27: 4564-4570 (2017)
- Wang, F; Jeon, KO; Salovich, JM; Macdonald, JD; Alvarado, J; Gogliotti, RD; Phan, J; Olejniczak, ET; Sun, Q; Wang, S; Camper, D; Yuh, JP; Shaw, JG; Sai, J; Rossanese, OW; Tansey, WP; Stauffer, SR; Fesik, SW J Med Chem 61: 5623-5642 (2018)
- Kallander, LS; Lu, Q; Chen, W; Tomaszek, T; Yang, G; Tew, D; Meek, TD; Hofmann, GA; Schulz-Pritchard, CK; Smith, WW; Janson, CA; Ryan, MD; Zhang, GF; Johanson, KO; Kirkpatrick, RB; Ho, TF; Fisher, PW; Mattern, MR; Johnson, RK; Hansbury, MJ; Winkler, JD; Ward, KW; Veber, DF; Thompson, SK J Med Chem 48: 5644-7 (2005)
- Zankel, TC; Isbell, SL; Ko, AA US Patent US10308607 (2019)
- Ko, B; Jang, Y; Kim, MH; Lam, TT; Seo, HK; Jeong, P; Choi, M; Kang, KW; Lee, SD; Park, JH; Kim, M; Han, SY; Kim, YC Eur J Med Chem 262:
- Li, Z; Liao, C; Ko, BC; Shan, S; Tong, EH; Yin, Z; Pan, D; Wong, VK; Shi, L; Ning, ZQ; Hu, W; Zhou, J; Chung, SS; Lu, XP Bioorg Med Chem Lett 14: 3507-11 (2004)
- Ko, CC; Chen, YJ; Chen, CT; Liu, YC; Cheng, FC; Hsu, KC; Chow, LP J Biol Chem 289: 22078-89 (2014)
- Shih, KC; Shiau, CW; Chen, TS; Ko, CH; Lin, CL; Lin, CY; Hwang, CS; Tang, CY; Chen, WR; Huang, JW Bioorg Med Chem Lett 21: 4490-7 (2011)
- Ratni, H; Ebeling, M; Baird, J; Bendels, S; Bylund, J; Chen, KS; Denk, N; Feng, Z; Green, L; Guerard, M; Jablonski, P; Jacobsen, B; Khwaja, O; Kletzl, H; Ko, CP; Kustermann, S; Marquet, A; Metzger, F; Mueller, B; Naryshkin, NA; Paushkin, SV; Pinard, E; Poirier, A; Reutlinger, M; Weetall, M; Zeller, A; Zhao, X; Mueller, L J Med Chem 61: 6501-6517 (2018)
- Nam, Y; Ryu, KD; Jang, C; Moon, YH; Kim, M; Ko, D; Chung, KS; Gandini, MA; Lee, KT; Zamponi, GW; Lee, JY Bioorg Med Chem 28: (2020)
- Lee, Y; Kim, H; Kim, H; Cho, HY; Jee, JG; Seo, KA; Son, JB; Ko, E; Choi, HG; Kim, ND; Kim, I J Med Chem 64: 6985-6995 (2021)
- Bardelle, C; Coleman, T; Cross, D; Davenport, S; Kettle, JG; Ko, EJ; Leach, AG; Mortlock, A; Read, J; Roberts, NJ; Robins, P; Williams, EJ Bioorg Med Chem Lett 18: 5717-21 (2009)
- Cheng, MC; Li, CY; Ko, HC; Ko, FN; Lin, YL; Wu, TS J Nat Prod 69: 1305-9 (2006)
- Hillmann, P; Ko, GY; Spinrath, A; Raulf, A; von Kügelgen, I; Wolff, SC; Nicholas, RA; Kostenis, E; Höltje, HD; Müller, CE J Med Chem 52: 2762-75 (2009)
- Lee, WG; Lee, SD; Cho, JH; Jung, Y; Kim, JH; Hien, TT; Kang, KW; Ko, H; Kim, YC J Med Chem 55: 3687-98 (2012)
- Cheng, MC; Li, CY; Ko, HC; Ko, FN; Lin, YL; Wu, TS J Nat Prod 69: 1305-9 (2006)
- Ng, LT; Ko, HH; Lu, TM Bioorg Med Chem 17: 4360-6 (2009)
- Choi, H; Park, HJ; Shin, JC; Ko, HS; Lee, JK; Lee, S; Park, H; Hong, S Bioorg Med Chem Lett 22: 2195-9 (2012)
- Dolan, BM; Duron, SG; Campbell, DA; Vollrath, B; Shankaranarayana Rao, BS; Ko, HY; Lin, GG; Govindarajan, A; Choi, SY; Tonegawa, S Proc Natl Acad Sci U S A 110: 5671-6
- Ham, J; Hwang, H; Kim, E; Kim, JA; Cho, SJ; Ko, J; Lee, W; Lee, J; Holla, H; Banerjee, J; Kim, S; Yang, I; Lee, HJ; Shin, K; Choi, H; Nam, SJ; Tak, J; Hahn, D; Oh, T; Won, DH; Lee, TG; Choi, J; Park, MS; Seok, C; Chin, J; Kang, H Eur J Med Chem 53: 190-202 (2012)
- Bhattarai, BR; Ko, JH; Shrestha, S; Kafle, B; Cho, H; Kang, JH; Cho, H Bioorg Med Chem Lett 20: 1075-7 (2010)
- Ko, K; Kim, HJ; Ho, PS; Lee, SO; Lee, JE; Min, CR; Kim, YC; Yoon, JH; Park, EJ; Kwon, YJ; Yun, JH; Yoon, DO; Kim, JS; Park, WS; Oh, SS; Song, YM; Cho, WK; Morikawa, K; Lee, KJ; Park, CH J Med Chem 61: 2949-2961 (2018)
- Lee, W; Ko, KR; Kim, HK; Lee, DS; Nam, IJ; Lim, S; Kim, S J Nat Prod 81: 1343-1356 (2018)
- Ko, KS; Steffey, ME; Brandvold, KR; Soellner, MB ACS Med Chem Lett 4: 779-783 (2013)
- Jeon, S; Ko, M; Lee, J; Choi, I; Byun, SY; Park, S; Shum, D; Kim, S Antimicrob Agents Chemother 64: (2020)
- Nam, M; Kim, T; Kwak, J; Seo, SH; Ko, MK; Lim, EJ; Min, SJ; Cho, YS; Keum, G; Baek, DJ; Lee, J; Pae, AN Eur J Med Chem 97: 245-58 (2015)
- Jackson, JJ; Shibuya, GM; Ravishankar, B; Adusumilli, L; Bradford, D; Brockstedt, DG; Bucher, C; Bui, M; Cho, C; Colas, C; Cutler, G; Dukes, A; Han, X; Hu, DX; Jacobson, S; Kassner, PD; Katibah, GE; Ko, MYM; Kolhatkar, U; Leger, PR; Ma, A; Marshall, L; Maung, J; Ng, AA; Okano, A; Pookot, D; Poon, D; Ramana, C; Reilly, MK; Robles, O; Schwarz, JB; Shakhmin, AA; Shunatona, HP; Sreenivasan, R; Tivitmahaisoon, P; Xu, M; Zaw, T; Wustrow, DJ; Zibinsky, M J Med Chem 65: 12895-12924 (2022)
- Srivastava, AS; Ko, S; Watterson, SH; Pattoli, MA; Skala, S; Cheng, L; Obermeier, MT; Vickery, R; Discenza, LN; D'Arienzo, CJ; Gillooly, KM; Taylor, TL; Pulicicchio, C; McIntyre, KW; Yip, S; Li, P; Sun, D; Wu, DR; Dai, J; Wang, C; Zhang, Y; Wang, B; Pawluczyk, J; Kempson, J; Zhao, R; Hou, X; Rampulla, R; Mathur, A; Galella, MA; Salter-Cid, L; Barrish, JC; Carter, PH; Fura, A; Burke, JR; Tino, JA ACS Med Chem Lett 11: 2195-2203 (2020)
- Lim, CJ; Woo, SE; Ko, SI; Lee, BH; Oh, KS; Yi, KY Bioorg Med Chem Lett 26: 4684-4686 (2016)
- Hwang, GJ; Jang, M; Son, S; Lee, B; Jang, JP; Lee, JS; Ko, SK; Hong, YS; Ahn, JS; Jang, JH J Nat Prod 84: 2420-2426 (2021)
- Rodgers, JD; Lam, PY; Johnson, BL; Wang, H; Li, R; Ru, Y; Ko, SS; Seitz, SP; Trainor, GL; Anderson, PS; Klabe, RM; Bacheler, LT; Cordova, B; Garber, S; Reid, C; Wright, MR; Chang, CH; Erickson-Viitanen, S Chem Biol 5: 597-608 (1998)
- Walpole, C; Ko, SY; Brown, M; Beattie, D; Campbell, E; Dickenson, F; Ewan, S; Hughes, GA; Lemaire, M; Lerpiniere, J; Patel, S; Urban, L J Med Chem 41: 3159-73 (1998)
- Lee, CC; Kuo, CJ; Ko, TP; Hsu, MF; Tsui, YC; Chang, SC; Yang, S; Chen, SJ; Chen, HC; Hsu, MC; Shih, SR; Liang, PH; Wang, AH J Biol Chem 284: 7646-55 (2009)
- Quang, TH; Ngan, NT; Ko, W; Kim, DC; Yoon, CS; Sohn, JH; Yim, JH; Kim, YC; Oh, H Bioorg Med Chem Lett 24: 5787-91 (2014)
- Ryu, CK; Kang, HY; Lee, SK; Nam, KA; Hong, CY; Ko, WG; Lee, BH Bioorg Med Chem Lett 10: 461-4 (2000)
- Kim, J; Kwon, J; Lee, D; Jo, S; Park, DS; Choi, J; Park, E; Hwang, JY; Ko, Y; Choi, I; Ju, MK; Ahn, J; Kim, J; Han, SJ; Kim, TH; Cechetto, J; Nam, J; Ahn, S; Sommer, P; Liuzzi, M; Lee, J Bioorg Med Chem Lett 24: 5473-7 (2015)
- Jeon, WS; Moon, K; Park, SH; Chun, H; Ko, YH; Lee, JY; Lee, ES; Samal, S; Selvapalam, N; Rekharsky, MV; Sindelar, V; Sobransingh, D; Inoue, Y; Kaifer, AE; Kim, K J Am Chem Soc 127: 12984-9 (2005)
- Kim, YK; Kwon, O; Park, H; Park, J; Choi, HG; Son, JB; Ko, E; Kim, SY; Lee, S; Kang, SY; Ko, YK; Park, J US Patent US11447480 (2022)
- Humblet, V; Misra, P; Bhushan, KR; Nasr, K; Ko, YS; Tsukamoto, T; Pannier, N; Frangioni, JV; Maison, W J Med Chem 52: 544-50 (2009)
- Suh, YG; Kim, NJ; Koo, BW; Lee, KO; Moon, SH; Shin, DH; Jung, JW; Paek, SM; Chang, DJ; Li, F; Kang, HJ; Le, TV; Chae, YN; Shin, CY; Kim, MK; Lim, JI; Ryu, JS; Park, HJ J Med Chem 51: 6318-33 (2008)
- Raddatz, P; Minck, KO; Rippmann, F; Schmitges, CJ J Med Chem 37: 486-97 (1994)
- Ghobish, SA; Mohamed, KO; Farag, N; Farag, DB RSC Med Chem 15: 293-308 (2024)
- Mohammed, KO; Nissan, YM Chem Biol Drug Des 84: 473-88 (2014)
- Bugge, S; Moen, IU; Sylte, KO; Sundby, E; Hoff, BH Eur J Med Chem 94: 175-94 (2015)
- Narayanan, D; Tran, KT; Pallesen, JS; Solbak, SMØ; Qin, Y; Mukminova, E; Luchini, M; Vasilyeva, KO; González Chichón, D; Goutsiou, G; Poulsen, C; Haapanen, N; Popowicz, GM; Sattler, M; Olagnier, D; Gajhede, M; Bach, A J Med Chem 65: 14481-14526 (2022)
- Yerdelen, KO; Koca, M; Anil, B; Sevindik, H; Kasap, Z; Halici, Z; Turkaydin, K; Gunesacar, G Bioorg Med Chem Lett 25: 5576-82 (2015)
- Lin, CH; Peng, YH; Coumar, MS; Chittimalla, SK; Liao, CC; Lyn, PC; Huang, CC; Lien, TW; Lin, WH; Hsu, JT; Cheng, JH; Chen, X; Wu, JS; Chao, YS; Lee, HJ; Juo, CG; Wu, SY; Hsieh, HP J Med Chem 52: 2618-22 (2009)
- Pontius, A; Krick, A; Mesry, R; Kehraus, S; Foegen, SE; Mu¨ller, M; Klimo, K; Gerha¨user, C; Ko¨nig, GM J Nat Prod 71: 1793-1799 (2008)
- Mattei, P; Boehringer, M; Di Giorgio, P; Fischer, H; Hennig, M; Huwyler, J; Koçer, B; Kuhn, B; Loeffler, BM; Macdonald, A; Narquizian, R; Rauber, E; Sebokova, E; Sprecher, U Bioorg Med Chem Lett 20: 1109-13 (2010)
- Bosnar, M; Kragol, G; Koštrun, S; Vujasinovic, I; Bošnjak, B; Bencetic Mihaljevic, V; Marušic Ištuk, Z; Kapic, S; Hrvacic, B; Brajša, K; Tavcar, B; Jelic, D; Glojnaric, I; Verbanac, D; Culic, O; Padovan, J; Alihodžic, S; Erakovic Haber, V; Spaventi, R J Med Chem 55: 6111-23 (2012)
- Schwardt, O; Rabbani, S; Hartmann, M; Abgottspon, D; Wittwer, M; Kleeb, S; Zalewski, A; Smieško, M; Cutting, B; Ernst, B Bioorg Med Chem 19: 6454-73 (2011)
- ZakoSek, M; Mihevc, SP; Majdic, G; Ko{hacek over (s)}ak, U; Gobec, S US Patent US20230331674 (2023)
- ChEMBL_2447201 Inhibition of human LYN
- ChEMBL_404537 (CHEMBL910920) Inhibition of LYN
- ChEMBL_424211 (CHEMBL908985) Inhibition of Lyn
- ChEMBL_437760 (CHEMBL905849) Inhibition of Lyn
- ChEMBL_444954 (CHEMBL894104) Inhibition of Lyn
- ChEMBL_446820 (CHEMBL897119) Inhibition of Lyn
- ChEMBL_447352 (CHEMBL895281) Inhibition of LYN
- ChEMBL_456444 (CHEMBL907533) Inhibition of Lyn
- ChEMBL_458461 (CHEMBL941778) Inhibition of LYN
- ChEMBL_474823 (CHEMBL950240) Inhibition of Lyn
- ChEMBL_480041 (CHEMBL927980) Inhibition of Lyn
- ChEMBL_489226 (CHEMBL989973) Inhibition of LYN
- ChEMBL_489691 (CHEMBL982996) Inhibition of LYN
- ChEMBL_498310 (CHEMBL973448) Inhibition of LYN
- ChEMBL_501541 (CHEMBL984181) Inhibition of Lyn
- ChEMBL_515223 (CHEMBL990378) Inhibition of Lyn
- ChEMBL_523124 (CHEMBL1007482) Inhibition of Lyn
- ChEMBL_531339 (CHEMBL990528) Inhibition of Lyn
- ChEMBL_534761 (CHEMBL986285) Inhibition of Lyn
- ChEMBL_535975 (CHEMBL987149) Inhibition of Lyn
- ChEMBL_537173 (CHEMBL992549) Inhibition of Lyn
- ChEMBL_563034 (CHEMBL1011864) Inhibition of LYN
- ChEMBL_570766 (CHEMBL1026941) Inhibition of LYN
- ChEMBL_575541 (CHEMBL1035538) Inhibition of LYN
- ChEMBL_597642 (CHEMBL1039941) Inhibition of Lyn
- ChEMBL_599162 (CHEMBL1049805) Inhibition of Lyn
- ChEMBL_599566 (CHEMBL1040178) Inhibition of Lyn
- ChEMBL_600780 (CHEMBL1037492) Inhibition of Lyn
- ChEMBL_606980 (CHEMBL1073307) Inhibition of LYN
- ChEMBL_611410 (CHEMBL1065741) Inhibition of LYN
- ChEMBL_612003 (CHEMBL1072210) Inhibition of LYN
- ChEMBL_612343 (CHEMBL1065595) Inhibition of Lyn
- ChEMBL_614210 (CHEMBL1105160) Inhibition of Lyn
- ChEMBL_620222 (CHEMBL1115857) Inhibition of LYN
- ChEMBL_623812 (CHEMBL1117095) Inhibition of LYN
- ChEMBL_625020 (CHEMBL1109688) Inhibition of Lyn
- ChEMBL_630548 (CHEMBL1108262) Inhibition of LYN
- ChEMBL_665078 (CHEMBL1260188) Inhibition of LYN
- ChEMBL_753057 (CHEMBL1798985) Inhibition of LYN
- ChEMBL_759689 (CHEMBL1811522) Inhibition of Lyn
- ChEMBL_772866 (CHEMBL1838269) Inhibition of LYN
- ChEMBL_786845 (CHEMBL1919763) Inhibition of LYN
- ChEMBL_793913 (CHEMBL1932886) Inhibition of LYN
- ChEMBL_803529 (CHEMBL1953277) Inhibition of Lyn
- ChEMBL_806863 (CHEMBL1958991) Inhibition of Lyn
- ChEMBL_828108 (CHEMBL2049847) Inhibition of LYN
- ChEMBL_828995 (CHEMBL2060645) Inhibition of LYN
- ChEMBL_861792 (CHEMBL2173385) Inhibition of Lyn
- ChEMBL_881214 (CHEMBL2211519) Inhibition of LYN
- ChEBML_1629294 Inhibition of LYN (unknown origin)
- ChEBML_221498 Inhibition of Lyn tyrosine kinase
- ChEMBL_1510625 (CHEMBL3607973) Inhibition of human LYN
- ChEMBL_1708086 (CHEMBL4059319) Inhibition of human LYN
- ChEMBL_2275492 Inhibition of LYN (unknown origin)
- ChEMBL_2282343 Inhibition of LYN (unknown origin)
- ChEMBL_2283234 Inhibition of LYN (unknown origin)
- ChEMBL_2335237 Inhibition of LYN (unknown origin)
- ChEMBL_2482735 Inhibition of Lyn (unknown origin)
- ChEMBL_2510982 Inhibition of LYN (unknown origin)
- ChEMBL_2525778 Inhibition of Lyn (unknown origin)
- ChEMBL_2544071 Inhibition of Lyn (unknown origin)
- ChEMBL_305891 (CHEMBL832906) Inhibition of Lyn kinase
- ChEMBL_328634 (CHEMBL863870) Inhibitory activity against lyn
- ChEMBL_473740 (CHEMBL936935) Inhibition of Lyn kinase
- ChEMBL_537189 (CHEMBL992565) Inhibition of Lyn kinase
- ChEMBL_545107 (CHEMBL1018049) Inhibition of human Lyn
- ChEMBL_589618 (CHEMBL1052171) Inhibition of human Lyn
- ChEMBL_619956 (CHEMBL1113998) Inhibition of LYN A
- ChEMBL_624375 (CHEMBL1107905) Inhibition of LYN A
- ChEMBL_628521 (CHEMBL1104643) Inhibition of LYN A
- ChEMBL_655053 (CHEMBL1244097) Inhibition of human Lyn
- ChEMBL_655442 (CHEMBL1244486) Binding affinity to LYN
- ChEMBL_813665 (CHEMBL2019867) Inhibition of human Lyn
- ChEBML_161954 Inhibition of Protein tyrosine kinase Lyn
- ChEBML_161960 Inhibition of Protein tyrosine kinase Lyn
- ChEBML_161961 Inhibition of Protein tyrosine kinase Lyn
- ChEBML_1684618 Inhibition of LYN B (unknown origin)
- ChEMBL_1348045 (CHEMBL3268709) Inhibition of Lyn (unknown origin)
- ChEMBL_1368031 (CHEMBL3300958) Inhibition of LYN (unknown origin)
- ChEMBL_1446770 (CHEMBL3381085) Inhibition of LYN (unknown origin)
- ChEMBL_1519163 (CHEMBL3624356) Inhibition of human recombinant Lyn
- ChEMBL_1542820 (CHEMBL3742710) Inhibition of LYN (unknown origin)
- ChEMBL_1544039 (CHEMBL3750411) Inhibition of LYN (unknown origin)
- ChEMBL_1654854 (CHEMBL4004220) Inhibition of LYN (unknown origin)
- ChEMBL_1720585 (CHEMBL4135585) Inhibition of Lyn (unknown origin)
- ChEMBL_1730346 (CHEMBL4145882) Inhibition of LYN (unknown origin)
- ChEMBL_1737126 (CHEMBL4152876) Inhibition of LYN (unknown origin)
- ChEMBL_1747157 (CHEMBL4181667) Inhibition of Lyn (unknown origin)
- ChEMBL_1776356 (CHEMBL4233348) Inhibition of LYN (unknown origin)
- ChEMBL_1778388 (CHEMBL4235380) Inhibition of LYN (unknown origin)
- ChEMBL_1870076 (CHEMBL4371243) Inhibition of LYN (unknown origin)
- ChEMBL_1983548 (CHEMBL4616954) Inhibition of LYN (unknown origin)
- ChEMBL_1995017 (CHEMBL4628912) Inhibition of Lyn (unknown origin)
- ChEMBL_2185704 (CHEMBL5097786) Inhibition of Lyn (unknown origin)
- ChEMBL_2568947 Inhibition of LYN A (unknown origin)
- ChEMBL_2571278 Inhibition of recombinant LYN (unknown origin)
- ChEMBL_343253 (CHEMBL861012) Inhibition of rat spleen Lyn
- ChEMBL_772087 (CHEMBL1837853) Inhibition of recombinant LYN kinase
- ChEMBL_941107 (CHEMBL2330904) Inhibition of Lyn (unknown origin)
- ChEMBL_944251 (CHEMBL2343901) Inhibition of LYN (unknown origin)
- ChEBML_221636 Inhibition of human p56 Lyn tyrosine kinase
- ChEMBL_161959 (CHEMBL769383) Inhibition of Protein tyrosine kinase Lyn
- ChEMBL_2066527 (CHEMBL4721780) Binding affinity to LYN (unknown origin)
- ChEMBL_2237739 (CHEMBL5151635) Inhibition of recombinant Lyn (unknown origin)
- ChEMBL_455237 (CHEMBL906398) Inhibition of Lyn by HTRF assay
- ChEMBL_469617 (CHEMBL934196) Inhibition of Lyn by HTRF assay
- ChEMBL_586970 (CHEMBL1037544) Binding constant for LYN kinase domain
- ChEMBL_621340 (CHEMBL1109433) Inhibition of Lyn after 1 hr
- ChEMBL_774342 (CHEMBL1908559) Binding constant for LYN kinase domain
- ChEMBL_221637 (CHEMBL823027) Inhibitory activity against p56 Lyn tyrosine kinase
- ChEMBL_801401 (CHEMBL1948131) Inhibition of LYN using ATP as substrate
- ChEMBL_847953 (CHEMBL2150253) Inhibition of LYN B after 5 mins
- ChEMBL_847954 (CHEMBL2150254) Inhibition of LYN A after 5 mins
- ChEMBL_1737121 (CHEMBL4152871) Inhibition of LYN (unknown origin) by FRET assay
- ChEMBL_2045485 (CHEMBL4700184) Inhibition of human LYN by kinase-profiling analysis
- ChEMBL_2538035 Inhibition of human LYN by discoverX kinome scan assay
- ChEMBL_478597 (CHEMBL934544) Inhibition of Lyn by luminescence based kinase assay
- ChEMBL_659486 (CHEMBL1248539) Binding affinity to Lyn by radioligand displacement assay
- ChEMBL_760762 (CHEMBL1815747) Inhibition of LYN by microfluidic mobility shift assay
- ChEMBL_535873 (CHEMBL994993) Inhibition of TEL fused Lyn-mediated proliferation of TEL-Lyn transformed mouse BA/F3 cells after 48 hrs by bright-glo luciferase assay
- ChEMBL_161953 (CHEMBL769207) Inhibition of Lyn kinase against 100 uM cdc2 substrate
- ChEMBL_443314 (CHEMBL892515) Inhibition of human Lyn kinase expressed in Sf9 cells
- ChEMBL_536666 (CHEMBL995057) Inhibition of Lyn in presence of 20 uM ATP
- ChEMBL_729720 (CHEMBL1697121) Inhibition of LYN after 60 mins by radiometric assay
- ChEMBL_1565895 (CHEMBL3789999) Inhibition of LYN (unknown origin) in presence of [gamma33P]ATP
- ChEMBL_1926315 (CHEMBL4429387) Inhibition of partial length human LYN expressed in bacterial system
- ChEMBL_2477775 Inhibition of human LYN incubated for 1 hr by HTRF assay
- ChEMBL_2544488 Inhibition of LYN (unknown origin) in presence of [gamma-33P]ATP
- ChEMBL_619937 (CHEMBL1113979) Inhibition of Tel-fused LYN expressed in mouse BAF3 cells
- ChEMBL_751372 (CHEMBL1785333) Inhibition of human LYN using poly[Glu:Tyr] by Hotspot assay
- ChEMBL_820939 (CHEMBL2038984) Inhibition of Tel-fused LYN in mouse BA/F3 cells
- ChEMBL_1546877 (CHEMBL3747980) Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate
- ChEMBL_2283236 Inhibition of LYN (unknown origin) at 1 uM by kinase-profiling analysis
- ChEMBL_2565098 Inhibition of LYN (unknown origin) Lys1 labeling site by KiNativ Profiling analysis
- ChEMBL_325069 (CHEMBL860805) Average Binding Constant for LYN; NA=Not Active at 10 uM
- ChEMBL_509002 (CHEMBL1006343) Inhibition of Tel-fused Lyn kinase-mediated mouse BaF3 cell proliferation
- ChEMBL_934835 (CHEMBL2319228) Inhibition of human LYN using poly[Glu:Tyr] (4:1) peptide substrate
- ChEMBL_1516624 (CHEMBL3620299) Inhibition of Lyn (unknown origin) after 40 mins by scintillation counting analysis
- ChEMBL_1546878 (CHEMBL3747981) Inhibition of human LYN B using poly[Glu:Tyr] (4:1) as substrate
- ChEMBL_1892790 (CHEMBL4394711) Inhibition of LYN (unknown origin) incubated for 60 mins by HTRF assay
- ChEMBL_2130170 (CHEMBL4839599) Inhibition of human full length recombinant LYN by radiometric scintillation counting analysis
- ChEMBL_2514898 Binding affinity to human LYN incubated for 45 mins by Kinobead based pull down assay
- ChEMBL_2517555 Inhibition of LYN (unknown origin) in the presence of [33P]ATP by kinase hotspot assay
- ChEMBL_772096 (CHEMBL1837862) Inhibition of LYN-mediated proliferation of mouse BAF3 cells transformed with TEL-Kinase construct
- ChEMBL_2476844 Inhibition of tetracycline-inducible FLAG-tagged human PARL stably transfected in HEK293T harboring FITR/PARL KO
- ChEMBL_1670305 (CHEMBL4020193) Inhibition of recombinant human LYN using TK-substrate-biotin after 40 mins by HTRF assay
- ChEMBL_2196460 (CHEMBL5108976) Inhibition of LYN (unknown origin) in presence of [gamma33P]-ATP and ATP by ELISA method
- ChEMBL_2436152 Inhibition of LYN (unknown origin) incubated for 60 mins in presence of ATP by KinaseGlo assay
- ChEMBL_788549 (CHEMBL1918489) Inhibition of LYN-A expressed in Sf9 cells after 60 mins by TR-FRET Assay
- ChEMBL_480051 (CHEMBL927990) Inhibition of mouse PKCtheta in KO cells assessed as blockade of anti CD28-stimulated IL2 production
- ChEMBL_1828603 (CHEMBL4328477) Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay
- ChEMBL_2065423 (CHEMBL4720676) Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay
- ChEMBL_827261 (CHEMBL2050660) Inhibition of Lyn B using ATP preincubated with compound for 5 mins measured after 10 mins
- ChEMBL_827264 (CHEMBL2050663) Inhibition of Lyn A using ATP preincubated with compound for 5 mins measured after 10 mins
- ChEMBL_1623197 (CHEMBL3865549) Inhibition of N-terminal his6-tagged recombinant full-length human LYN expressed in baculovirus infected Sf21 cells
- ChEMBL_1699210 (CHEMBL4050192) Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate in presence of [gamma-32P]ATP
- ChEMBL_1828604 (CHEMBL4328478) Inhibition of human LYN-B using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay
- ChEMBL_1909763 (CHEMBL4412209) Inhibition of human LYN-B using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay
- ChEMBL_2065421 (CHEMBL4720674) Inhibition of human LYN-B using poly[Glu:Tyr] (4:1) as substrate by [gamma-33P]-ATP assay
- ChEMBL_2563684 Inhibition of LYN (unknown origin) incubated for 1 hr by colloidal coomassie staining based LC-MS/MS analysis
- ChEMBL_2573574 Inhibition of recombinant LYN (unknown origin) preincubated for 1 hr in presence of ATP by Z-Lyte assay
- ChEMBL_745850 (CHEMBL1776220) Inhibition of LYN assessed as [33P]gamma-ATP incorporation into substrate after 60 mins by gamma counting
- ChEMBL_886741 (CHEMBL2210055) Inhibition of recombinant LYN after 1 hr by scintillation counter analysis in presence of gamma-[33P]ATP
- ChEMBL_2284045 Inhibition of human JAK3 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_2284046 Inhibition of human JAK2 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_2284047 Inhibition of human JAK1 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_2154863 (CHEMBL5039523) Agonist activity at STING KO human THP-1 dual cells incubated for 20 hrs by luciferase reporter gene assay
- ChEMBL_1809941 (CHEMBL4309401) Inhibition of human JAK1 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_1809942 (CHEMBL4309402) Inhibition of human JAK2 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_1809943 (CHEMBL4309403) Inhibition of human JAK3 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_1841034 (CHEMBL4341333) Inhibition of human JAK1 kinase-domain using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_1841035 (CHEMBL4341334) Inhibition of human JAK2 kinase-domain using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_1841036 (CHEMBL4341335) Inhibition of human JAK3 kinase-domain using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr by ELISA
- ChEMBL_2059265 (CHEMBL4714266) Inhibition of human JAK1 using Biotin-Lyn-Substrate-2 in presence of 1 mM ATP by phosphotyrosine-specific ELISA
- ChEMBL_2059266 (CHEMBL4714267) Inhibition of human JAK2 using Biotin-Lyn-Substrate-2 in presence of 1 mM ATP by phosphotyrosine-specific ELISA
- ChEMBL_2059267 (CHEMBL4714268) Inhibition of human JAK3 using Biotin-Lyn-Substrate-2 in presence of 1 mM ATP by phosphotyrosine-specific ELISA
- ChEMBL_2059268 (CHEMBL4714269) Inhibition of human Tyk2 using Biotin-Lyn-Substrate-2 in presence of 1 mM ATP by phosphotyrosine-specific ELISA
- ChEMBL_2567821 Inhibition of human LYN using poly (Glu, Tyr)4:1 as substrate in presence of [gamma33P]-ATP by HotSpot assay
- ChEMBL_824235 (CHEMBL2044290) Inhibition of LYN incubated for 30 mins prior to substrate addition study done at apparent ATP Km for enzyme
- ChEMBL_2322358 Agonist activity at STING in human STHP1-Dual KO-STING cells incubated for 20 hrs by Quanti-luc reagent based assay
- ChEMBL_2354137 Agonist activity at STING in PMA-differentiated human THP1-Dual KO-STING cells incubated for 24 hrs by QUANTI-Blue assay
- ChEMBL_1505205 (CHEMBL3594790) Inhibition of human JAK2 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA method
- ChEMBL_1505206 (CHEMBL3594791) Inhibition of human JAK1 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA method
- ChEMBL_1505207 (CHEMBL3594792) Inhibition of human JAK3 kinase domain using biotin-Lyn-substrate-2 as substrate incubated for 1 hr by ELISA method
- ChEMBL_1802632 (CHEMBL4274924) Inhibition of Lyn (unknown origin) using Src-family kinase bisamide rhodamine 110 peptide substrate after 1 hr by fluorescence assay
- ChEMBL_2065003 (CHEMBL4720256) Inhibition of recombinant human LYN using biotinyl-beta Abeta-Abeta AKVEKIGEGTYGVVYK as substrate incubate for 120 mins by LANCE assay
- ChEMBL_2428436 Inhibition of human JAK1 using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hrs in presence of ATP by ELISA
- ChEMBL_2428437 Inhibition of human JAK2 using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hrs in presence of ATP by ELISA
- ChEMBL_2428438 Inhibition of human JAK3 using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hrs in presence of ATP by ELISA
- ChEMBL_787269 (CHEMBL1919047) Inhibition of LYN-A expressed in Sf9 cells preincubated for 60 mins measured after 60 mins by TR-FRET Assay
- ChEMBL_2578626 Inhibition of GST tagged Lyn (unknown origin) assessed as inhibition of phosphorylation preincubated for 5 mins followed by [gamma-32P]ATP addition
- Inhibition Assay The following Table shows the activity of selected compounds of this invention in the BTK, TEC, BLK, LYN, LCK inhibition assay.
- ChEMBL_1505110 (CHEMBL3594575) Inhibition of human JAK3 kinase domain using biotin-Lyn-substrate-2 as substrate and ATP incubated for 1 hr by ELISA method
- ChEMBL_1505111 (CHEMBL3594576) Inhibition of human JAK2 kinase domain using biotin-Lyn-substrate-2 as substrate and ATP incubated for 1 hr by ELISA method
- ChEMBL_1505112 (CHEMBL3594577) Inhibition of human JAK1 kinase domain using biotin-Lyn-substrate-2 as substrate and ATP incubated for 1 hr by ELISA method
- ChEMBL_1649215 (CHEMBL3998349) Inhibition of recombinant human Lyn using peptide substrate poly[Glu:Tyr] (4:1) in presence of [33-P]ATP by kinase hotspot assay
- ChEMBL_2473762 Inhibition of PRMT5 in human HCT-116 cells with MTAP KO assessed as decrease in SMDA level incubated for 48 hrs by immunofluorescence analysis
- ChEMBL_1664163 (CHEMBL4013844) Inhibition of human LYN-B preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis method
- ChEMBL_1664164 (CHEMBL4013845) Inhibition of human LYN-A preincubated for 15 mins followed by peptide substrate addition measured after 3 hrs by caliper capillary electrophoresis method
- ChEMBL_1926454 (CHEMBL4429526) Inhibition of human JAK3 assessed as reduction in phosphorylation of Biotin-Lyn-Substrate-2 after 1 hr in presence of ATP by ELISA
- ChEMBL_1926455 (CHEMBL4429527) Inhibition of human JAK1 assessed as reduction in phosphorylation of Biotin-Lyn-Substrate-2 after 1 hr in presence of ATP by ELISA
- ChEMBL_1926456 (CHEMBL4429528) Inhibition of human JAK2 assessed as reduction in phosphorylation of Biotin-Lyn-Substrate-2 after 1 hr in presence of ATP by ELISA
- ChEMBL_1986201 (CHEMBL4619607) Inhibition of human LYN using poly(Glu,Tyr)4:1 substrate and [gamma-33P]-ATP incubated for 40 mins by scintillation counting method
- ChEMBL_2462507 Inhibition of LYN (unknown origin) preincubated for 20 mins followed by 33P-ATP addition and measured after 2 hrs by P81 filter-binding method
- ChEMBL_1909791 (CHEMBL4412237) Inhibition of Lyn B (unknown origin) preincubated for 10 mins followed by substrate addition and measured after 1 hr by ADP-Glo luminescence assay
- ChEMBL_2125046 (CHEMBL4834279) Inhibition of LYN (unknown origin) expressed in mouse BaF3 cells assessed as reduction in cell proliferation incubated for 48 hrs by cell proliferation assay
- ChEMBL_1861462 (CHEMBL4362318) Inhibition of LYN (unknown origin) using KVEKIGEGTYGVVYK as substrate in presence of [gamma33P]ATP as substrate in presence of [gamma33P]ATP by scintillation counting method
- ChEMBL_1922046 (CHEMBL4424891) Inhibition of full-length human N-terminal His6-tagged LYN expressed in baculovirus infected Sf21 insect cells using Poly (Glu4-Tyr) (4:1) as substrate
- ChEMBL_2527341 Inhibition of LYN A (unknown origin) in presence of ATP Km treated at 2 hrs followed by IGF1 ligand addition for 15 mins by ELISA analysis
- ChEMBL_2527342 Inhibition of LYN B (unknown origin) in presence of ATP Km treated at 2 hrs followed by IGF1 ligand addition for 15 mins by ELISA analysis
- ChEMBL_2160781 (CHEMBL5045531) Agonist activity at STING in human THP1 Dual KO-STING cells assessed as IRF reporter activation incubated for 20 hrs by quanti-blue SEAP reporter gene assay
- ChEMBL_2160782 (CHEMBL5045532) Agonist activity at STING in mouse RAW-Lucia ISG-KO-STING cells assessed as IRF reporter activation incubated for 20 hrs by quanti-blue SEAP reporter gene assay
- ChEMBL_2160784 (CHEMBL5045534) Agonist activity at STING in human THP1-Dual KO-STING cells assessed as NF-kappaB reporter activation incubated for 20 hrs by quanti-blue SEAP reporter gene assay
- ChEMBL_1498722 (CHEMBL3583135) Inhibition of Tel-fused LYN (unknown origin) expressed in mouse BA/F3 cells after 48 hrs by luciferase reporter gene assay in absence of recombinant mouse IL3
- ChEMBL_1846524 (CHEMBL4347065) Inhibition of human recombinant LYN using biotinyl-beta amyloid beta amyloid beta AKVEKIGEGTYGVVYK as substrate measured after 120 mins in the presence of ATP by HTRF assay
- ChEMBL_1972992 (CHEMBL4605810) Inhibition of LYN (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-luciferase reporter gene assay
- ChEMBL_2085769 (CHEMBL4767032) Inhibition of recombinant human Lyn using poly(Glu, Tyr) 4:1 as substrate after 40 mins in presence of [gamma-33ATP] by scintillation counting based radiometry assay
- ChEMBL_1789706 (CHEMBL4261440) Inhibition of recombinant full length human Lyn using poly(Glu, Tyr) 4:1 as substrate after 40 mins in presence of [gamma-33P]-ATP by scintillation counting analysis
- ChEMBL_1877988 (CHEMBL4379382) Inhibition of recombinant full length human Lyn using poly(Glu, Tyr) 4:1 as substrate after 40 mins in presence of [gamma-33ATP] by radiometric scintillation counting analysis
- ChEMBL_2155048 (CHEMBL5039708) Inhibition of human LYN using poly [Glu:Tyr] as substrate incubated for 30 mins followed by 33P-ATP addition and measured after 2 hrs by liquid scintillation counter method
- ChEMBL_2543811 Inhibition of human wild type LYN using PolyEY as substrate preincubated for 2 hrs followed by ATP addition and measured every 2 mins for 2.5 hrs by spectrophotometric analysis
- ChEMBL_1779475 (CHEMBL4236467) Inhibition of recombinant human N-terminal His-tagged JAK3 catalytic domain (795 to 1124 residues) expressed in baculovirus expression system using Biotin-Lyn-Substrate2 after 1 hr by ELISA
- ChEMBL_1779476 (CHEMBL4236468) Inhibition of recombinant human N-terminal GST-tagged JAK1 catalytic domain (850 to 1154 residues) expressed in baculovirus expression system using Biotin-Lyn-Substrate2 after 1 hr by ELISA
- ChEMBL_1779477 (CHEMBL4236469) Inhibition of recombinant human N-terminal His-tagged JAK2 catalytic domain (826 to 1132 residues) expressed in baculovirus expression system using Biotin-Lyn-Substrate2 after 1 hr by ELISA
- ChEMBL_1779478 (CHEMBL4236470) Inhibition of recombinant human N-terminal GST-tagged TYK2 catalytic domain (871 to 1187 residues) expressed in baculovirus expression system using Biotin-Lyn-Substrate2 after 1 hr by ELISA
- ChEMBL_1989431 (CHEMBL4623166) Inhibition of LYN (unknown origin) using FAM-labeled peptide and ATP as substrate preincubated for 10 mins followed by substrate addition measured after 1 hr by mobility shift assay
- ChEMBL_2084882 (CHEMBL4766145) Inhibition of human full length recombinant LYN using poly(Glu,Tyr)4:1 as substrate incubated for 40 mins in presence of [gamma33P-ATP] by radiometric scintillation counting analysis
- ChEMBL_2336323 Inhibition of human JAK1 using Biotin-Lyn-Substrate-2 as substrate assessed as reduction in rate of substrate phosphorylation incubated for 1 hr in presence of ATP by ELISA method
- ChEMBL_2336324 Inhibition of human JAK2 using Biotin-Lyn-Substrate-2 as substrate assessed as reduction in rate of substrate phosphorylation incubated for 1 hr in presence of ATP by ELISA method
- ChEMBL_2336325 Inhibition of human JAK3 using Biotin-Lyn-Substrate-2 as substrate assessed as reduction in rate of substrate phosphorylation incubated for 1 hr in presence of ATP by ELISA method
- ChEMBL_2526413 Inhibition of LYN in human HeLa cells lysate pre incubated for 15 mins followed by ATP acyl phosphate probe addition and measured after 10 mins by LC-MS/MS analysis
- ChEMBL_2578591 Inhibition of GST tagged Lyn (unknown origin) assessed as inhibition of phosphorylation using PTK biotinylated peptide substrate 2 as substrate preincubated for 5 mins followed by [gamma-32P]ATP addition
- ChEMBL_1823007 (CHEMBL4322771) Binding affinity to recombinant full-length N-terminal His-FLAG-GST-tagged LYN (unknown origin) expressed in baculovirus infected Sf9 insect cells incubated for 1 hr by TR-FRET assay
- ChEMBL_1824182 (CHEMBL4323946) Inhibition of recombinant full length human LYN using poly(Glu, Tyr) 4:1 as substrate incubated for 40 mins in presence of [gamma33P]-ATP by scintillation counting based radiometry assay
- ChEBML_1970702 Inhibition of recombinant full length human GST-tagged LYN-A expressed in insect cells using tyrosine-2 peptide as substrate incubated for 60 mins in presence of ATP by Z'-LYTE assay
- ChEMBL_2583267 Inhibition of human LYN using poly[Glu:Tyr] (4:1) as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition and measured after 120 mins by radiometric Hot-SpotSM Kinase assay
- ChEMBL_1970702 (CHEMBL4603520) Inhibition of recombinant full length human GST-tagged LYN-A expressed in insect cells using tyrosine-2 peptide as substrate incubated for 60 mins in presence of ATP by Z'-LYTE assay
- ChEMBL_2583268 Inhibition of human LYN B using poly[Glu:Tyr] (4:1) as substrate preincubated for 20 mins followed by [gamma-33P]-ATP addition and measured after 120 mins by radiometric Hot-SpotSM Kinase assay
- ChEMBL_1706902 (CHEMBL4058135) Inhibition of recombinant human full length N-terminal His6-tagged LYN expressed in Sf21 insect cells preincubated for 5 mins followed by His-tagged Rb truncated protein substrate addition after 30 to 60 mins in presence of ATP by fluorescence assay
- ChEMBL_2331827 Inhibition of N-terminal GST tagged catalytic domain human JAK1 (850 to 1154 end residues) expressed in Sf21 insect cells using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr followed by substrate addition in presence of ATP by ELISA method
- ChEMBL_2331828 Inhibition of N-terminal His-tagged catalytic domain human JAK2 (826 to 1132 end residues) expressed in Sf21 insect cells using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr followed by substrate addition in presence of ATP by ELISA method
- ChEMBL_2331829 Inhibition of N-terminal His-tagged catalytic domain human JAK3 (795 to 1124 residues) expressed in Sf21 insect cells using Biotin-Lyn-Substrate-2 as substrate incubated for 1 hr followed by substrate addition in presence of ATP by ELISA method
- Lyn Enzyme Activity Lyn enzyme (Millipore catalog #14-510), is diluted to 250 mU/mL in KR buffer (10 mM Tris-HCl, 10 mM MgCl2, 0.01% Tween-20, 0.05% NaN3, 1 mM DTT, 2 mM MnCl2, pH 7.2).Serial dilution log 10 from 2 mM to 63.2 nM of test compounds are made in 100% DMSO. The dilutions in DMSO are then diluted 50-fold in KR-buffer of which 5 μl is used in the assay, leading to a final compound concentration range in the assay from 10 μM to 0.316 nM.5 μL/well of test compound in KR buffer (final DMSO concentration in the assay is 1%) is mixed with 5 μl/well of 250 mU/mL Lyn enzyme (final concentration in the assay is 62.5 mU/mL). Test compounds and Lyn enzyme are pre-incubated 60 minutes at room temperature, before adding 5 μL/well of 400 nM Fluorescin labeled substrate peptide (Blk/Lyntide substrate, e.g. #R7188/#R7233, Molecular Devices) in KR-buffer. Final peptide substrate concentration in assay is 100 nM. The kinase assay is started by adding 5 μL/well of 8 μM ATP in KR-buffer (final ATP concentration is 2 μM ATP, Km ATP in Lyn IMAP assay). Following incubation for 2 h at room temperature the enzyme reaction is stopped by adding 40 μL/well IMAP Progressive Binding Solution (according to suppliers (Molecular Devices) protocol using 75% 1× buffer A and 25% 1× buffer B with 1:600 Progressive Binding Solution). After 60 min incubation at room temperature in the dark the FP signal is read. Fluorescence at 535 nm is measured using parallel and perpendicular filters to determine differences in rotation due to binding of the phosphorylated substrate peptide to the beads. Values are calculated as percentage of the difference in readout (ΔmPi) of the controls with and without ATP. EC50 values are determined by curve fitting of the experimental results using Activity Base.
- Lyn Enzyme Activity Lyn enzyme activity is measured using the IMAP (immobilized metal ion affinity-based fluorescence polarization) assay as outlined below.Lyn enzyme (Millipore catalog #14-510), is diluted to 250 mU/mL in KR buffer (10 mM Tris-HCl, 10 mM MgCl2, 0.01% Tween-20, 0.05% NaN3, 1 mM DTT, 2 mM MnCl2, pH 7.2).Serial dilution log 10 from 2 mM to 63.2 nM of test compounds are made in 100% DMSO. The dilutions in DMSO are then diluted 50-fold in KR-buffer of which 5 μl is used in the assay, leading to a final compound concentration range in the assay from 10 μM to 0.316 nM.5 μL/well of test compound in KR buffer (final DMSO concentration in the assay is 1%) is mixed with 5 μl/well of 250 mU/mL Lyn enzyme (final concentration in the assay is 62.5 mU/mL). Test compounds and Lyn enzyme are pre-incubated 60 minutes at room temperature, before adding 5 μL/well of 400 nM Fluorescin labeled substrate peptide (Blk/Lyntide substrate, e.g. #R7188/#R7233, Molecular Devices) in KR-buffer. Final peptide substrate concentration in assay is 100 nM. The kinase assay is started by adding 5 μL/well of 8 μM ATP in KR-buffer (final ATP concentration is 2 μM ATP, Km ATP in Lyn IMAP assay). Following incubation for 2 h at room temperature the enzyme reaction is stopped by adding 40 μL/well IMAP Progressive Binding Solution (according to suppliers (Molecular Devices) protocol using 75% 1× buffer A and 25% 1× buffer B with 1:600 Progressive Binding Solution). After 60 min incubation at room temperature in the dark the FP signal is read. Fluorescence at 535 nm is measured using parallel and perpendicular filters to determine differences in rotation due to binding of the phosphorylated substrate peptide to the beads. Values are calculated as percentage of the difference in readout (ΔmPi) of the controls with and without ATP. EC50 values are determined by curve fitting of the experimental results using Activity Base.
- Enzyme Activity Assay Lyn enzyme activity is measured using the IMAP (immobilized metal ion affinity-based fluorescence polarization) assay as outlined below.Lyn enzyme (Millipore catalog #14-510), is diluted to 250 mU/mL in KR buffer (10 mM Tris-HCl, 10 mM MgCl2, 0.01% Tween-20, 0.05% NaN3, 1 mM DTT, 2 mM MnCl2, pH 7.2).Serial dilution log 10 from 2 mM to 63.2 nM of test compounds are made in 100% DMSO. The dilutions in DMSO are then diluted 50-fold in KR-buffer of which 5 μl is used in the assay, leading to a final compound concentration range in the assay from 10 μM to 0.316 nM.5 μL/well of test compound in KR buffer (final DMSO concentration in the assay is 1%) is mixed with 5 μl/well of 250 mU/mL Lyn enzyme (final concentration in the assay is 62.5 mU/mL). Test compounds and Lyn enzyme are pre-incubated 60 minutes at room temperature, before adding 5 μL/well of 400 nM Fluorescin labeled substrate peptide (Blk/Lyntide substrate, e.g. #R7188/#R7233, Molecular Devices) in KR-buffer. Final peptide substrate concentration in assay is 100 nM. The kinase assay is started by adding 5 μL/well of 8 μM ATP in KR-buffer (final ATP concentration is 2 μM ATP, Km ATP in Lyn IMAP assay). Following incubation for 2 h at room temperature the enzyme reaction is stopped by adding 40 μL/well IMAP Progressive Binding Solution (according to suppliers (Molecular Devices) protocol using 75% 1× buffer A and 25% 1× buffer B with 1:600 Progressive Binding Solution). After 60 min incubation at room temperature in the dark the FP signal is read. Fluorescence at 535 nm is measured using parallel and perpendicular filters to determine differences in rotation due to binding of the phosphorylated substrate peptide to the beads. Values are calculated as percentage of the difference in readout (ΔmPi) of the controls with and without ATP.
- Kinase Assay Btk kinase activity was determined using a homogenous time resolved fluorescence (HTRF) methodology. Measurements were performed in a reaction volume of 15 μL using 384-well assay plates. Kinase enzyme, inhibitor, ATP and 1 μM peptide substrate were incubated in a reaction buffer compose of Hepes 50 mM (pH7.0), NaN3 0.02%, BSA 0.01%, Orthocanadate 0.1 mM. After one hour, the kinase reaction was quenched by the addition of Eμ-labeled antibody and XL-665 in 1× Detection buffer containing 60 mM EDTA (Cisbio), and the mixture was allowed to incubate for one hour. The HTRF signal was measured on a multimode plate reader (EnVision Multilabel Reader, Perkin Elmer) with an excitation wavelength (λEx) of 330 nm and detection wavelengths (λEm) of 615 and 665 nrm. Activity was determined by the ratio of the fluorescence at 665 nm to that at 615 nm. For each compound, enzyme activity as measured at various concentrations of compound, Negative control reactions were performed in the absence of inhibitor in two replicates and eight no enzyme controls were used to determine baseline fluorescence levels.For LYN assay, [ATP]=20 μM, LYN=0.12 n M. For LCK assay, [ATP]=20 μM, LCK=0.2 nM. For BLK assay, [ATP]=20 μM, BLK=0.6 n M.
- Kinases Assay Btk kinase activity was determined using a homogenous time resolved fluorescence (HTRF) methodology. Measurements were performed in a reaction volume of 15 μL using 384-well assay plates. Kinase enzyme, inhibitor, ATP and 1 μM peptide substrate were incubated in a reaction buffer compose of Hepes50 mM (pH7.0), NaN3 0.02%, BSA 0.01%, Orthocanadate 0.1 mM. After one hour, the kinase reaction was quenched by the addition of E t-labeled antibody and XL-665 in 1×Detection buffer containing 60 mM EDTA (Cisbio), and the mixture was allowed to incubate for one hour. The HTRF signal was measured on a multimode plate reader (EnVision Multilabel Reader, Perkin Elmer) with an excitation wavelength (λEx) of 330 nm and detection wavelengths (λEm) of 615 and 665 nm. Activity was determined by the ratio of the fluorescence at 665 nm to that at 615 nm. For each compound, enzyme activity as measured at various concentrations of compound, Negative control reactions were performed in the absence of inhibitor in two replicates and eight no enzyme controls were used to determine baseline fluorescence levels. IC50s were obtained according to the equation:Y=100/(1+10{circumflex over ( )}((Log IC50−X)*HillSlope)).For BTK assay, [ATP]=80 μM, BTK=3.4 nM.For LYN assay, [ATP]=20 μM, LYN=0.12 n M. For LCK assay, [ATP]=20 μM, LCK=0.2 nM. For BLK assay, [ATP]=20 μM, BLK=0.6 n M.
- Determination of the value of binding affinity constant (KO between the compound and BRD4 BD2 protein The purity of BRD4 BD2 protein used in the experiment was greater than 95%, and the protein concentration was 46.33 uM. The 96-well plate was purchased from Corning (black, #3694). The multifunctional microplate reader was a product of TECAN, model: SPARK 10M. Buffer: 100 mM potassium phosphate (pH 6.5), 2% ethylene glycol (Sigma) and 0.01% Trition X-100 (Sigma). The experimental water was Millipore-Q pure water.The Ki value of the compound and BRD4 BD2 protein was measured according to the FP test procedures for detecting the Ki value of the compound and BRD4 BD1 protein except that the BRD4 BD1 protein was replaced with the BRD4 BD2 protein.
- In Vitor Src Tyrosine Kinase Activity Assay Kinase activity (Src-family kinases, Hck, Lyn, Fyn, and c-Src) and the effect of the molecules were determined by ProFluor Src-Family Kinase Assay Kit (Promega). A titration assay was performed for each kinase to determine the amount of the enzyme that results in approximately 80% phosphorylation as suggested by the manufacturer. The compounds were dissolved in 10% DMSO and tested at 1, 10 and 100 μM concentrations. Briefly, the molecules were mixed with a reaction buffer that included a specific substrate for Src-family kinases (R110), a control substrate and the kinase. The reaction was initiated by the addition of ATP. After incubating the 96-well reaction plate at 22°C for 1 h, protease solution was added to each well and the plate was incubated for 1 h. The fluorescence of the liberated R110 was read at an excitation wavelength of 485 nm and emission wavelength of 530 nm.
- Determination of the value of binding affinity constant (KO between the compound and BRD4 BD1 protein The purity of BRD4 BD1 protein used in the experiment was greater than 95%, and the protein concentration was 43.4 uM. The 96-well plate was purchased from Corning (black, #3694). The multifunctional microplate reader was a product of TECAN, model: SPARK 10M. Buffer: 100 mM potassium phosphate (pH 6.5), 2% ethylene glycol (Sigma) and 0.01% Trition X-100 (Sigma). The experimental water was Millipore-Q pure water.The specific experimental steps were as follows.First, the compound to be tested was dissolved in ethylene glycol to prepare into a 10 mM standard stock solution. Subsequently, the standard stock solution of the compound to be tested was diluted into a working sample solution with the buffer in an EP tube and ready for use. The concentration of the prepared working sample solution was 5 times of the highest sample concentration required on the test plate (5×test compound solution).40 λL of a 5× test compound solution of a sample A was added to wells B1-B3 of a 96-well plate, and 40 μL of a 5× test compound solution of a sample B was added to wells B7-B9 of the 96-well plate, respectively. 20 uL of the buffer was added to the remaining wells, except for wells B1-B3 and B7-B9. Then, 20 uL of a solution was taken from wells B1-B3 to C1-C3, and this 2-fold dilution was repeated from C1-C3 until H4-H6; in the same way, 20 uL of a solution was taken from B7-B9 to C7-C9, this 2-fold dilution was repeated from C7-C9 until H10-H12. Finally, 80 uL of a mixed solution containing 2.5 nM Tracer and 37.5 nM BRD4 BD1 protein was added to each well.
- Kinase Inhibition Assays The aim of this experiment is to detect the inhibitory activity of the compounds of the present invention against in vitro protein kinases using isotope labeling (labeled gamma phosphate groups on ATP). Kinase inhibition profiles were determined using KinaseProfiler services provided by Euro fins, and ATP concentrations used are the Km of corresponding kinases. In this study, we examined Abl (T315I) (h), ALK (h), ARK5 (h), Axl (h), Blk (h), Bmx (h), BTK (h), B-Raf (H), ckit (h), cSRC (h), CDK7, CHK1 (h), c-RAF (h), DDR2 (h), EGFR (h), EphA1 (h), EphA2 (h), EphA8 (h (H), FGF (h), Ft (h), Ft (h), F (h), F (h), Ft (h), Hb (h), ErbB2 (h), FAK (h) (H), JK3 (h), IKKalpha (h), IKKbeta (h), Itk (h), JAK3 (h), JNK11 (h), KDR (h), Lyn (h), MAPK1 (h), MEK1 (h (H), PKA (h), PKB [alpha](h), PKB [beta] (h), PKC [alpha] (h), Ret (H), RIPK2 (h), Src (1-530) (h), TAK1 (h), TBK1 (h), Tec (h) activated, Tie2 (h), TrkA (h), ULK3 (h) Yes (h), PI3 Kinase a (h) and other kinase in vitro inhibitory activity. The kinase inhibitory activity of the test compound is expressed as IC50 (half inhibitory concentration) or the inhibitory rate of the test compound at a concentration of 10 ??M for kinase activity. IC50 values can be obtained by calculating the inhibition rate of the kinase activity by the test compound at a series of different concentrations. The assay was as follows: In a reaction tube, the buffer (8 mM MOPS, pH 7.0, 0.2 mM EDTA, 10 mM MnCl2), the kinase to be tested (5-10 mU), the substrate to be tested kinase, 10 mM acetic acid Magnesium and gamma 33P-ATP solutions, as well as different concentrations of the test compound. MgATP was then added to the reaction to initiate the enzymatic reaction and incubated at room temperature for 40 minutes. The reaction was finally quenched with 5 ul of 3% phosphate buffer and 10 uL of the reaction solution was titrated onto a filtermat A membrane, washed three times with 75 mM phosphate solution for 5 minutes each, and washed again with methanol. Finally, the filtermat A film is dried and scintigraphized, and the size of the scintillation count reflects the degree to which the substrate is phosphorylated, thereby demonstrating that the kinase activity is inhibited. Among them, the percentage of the remaining active protein=the active protein of the experimental group the active protein of the control group 100%.
 CHEMBL1159714 Ko 707 BDBM50421719
CHEMBL1159714 Ko 707 BDBM50421719 3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester BDBM50305083 3-((3S,6S,12aS)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester Ko143 Ko-143 US9695174, Ko143 CHEMBL488910
3-((3S,6S)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester BDBM50305083 3-((3S,6S,12aS)-6-Isobutyl-9-methoxy-1,4-dioxo-1,2,3,4,6,7,12,12a-octahydro-pyrazino[1',2':1,6]pyrido[3,4-b]indol-3-yl)-propionic acid tert-butyl ester Ko143 Ko-143 US9695174, Ko143 CHEMBL488910