 US20240190875, Example Ghrelin BDBM21940 Ghrelin
US20240190875, Example Ghrelin BDBM21940 Ghrelin GHRELIN BDBM50366689
GHRELIN BDBM50366689 BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy
BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy BDBM85063 Melatonin,6-Cl
BDBM85063 Melatonin,6-Cl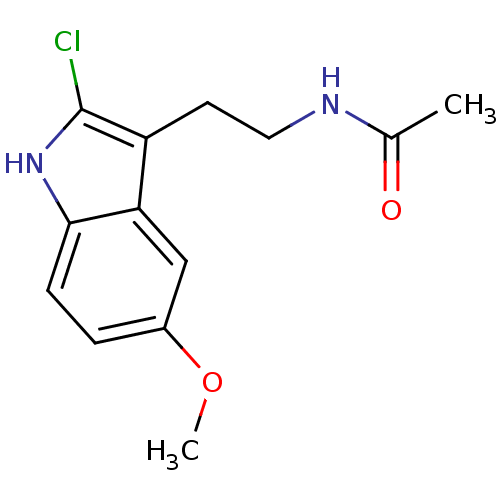 Melatonin,2-Chloro BDBM85236
Melatonin,2-Chloro BDBM85236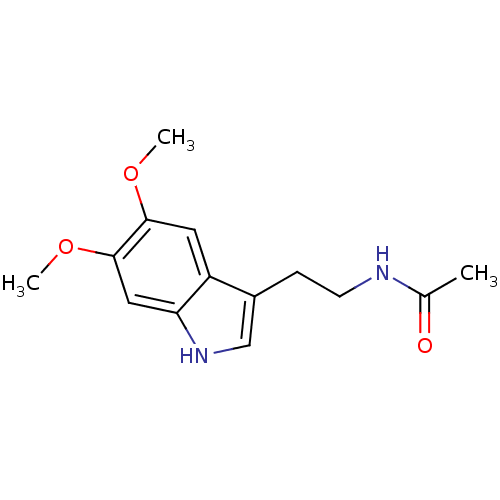 melatonin, 6-Methoxy BDBM82556
melatonin, 6-Methoxy BDBM82556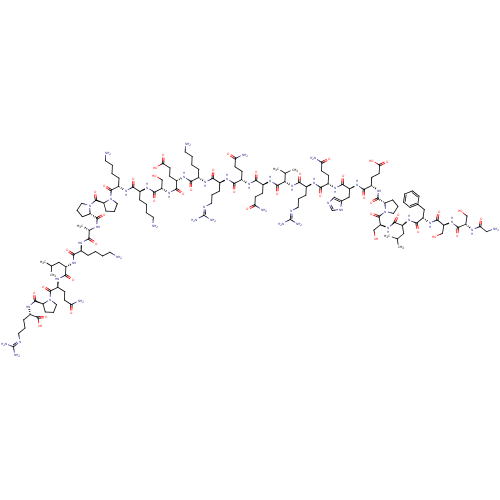 CHEMBL428282 Des-acyl ghrelin BDBM50163757
CHEMBL428282 Des-acyl ghrelin BDBM50163757 BDBM675521 US20240158438, SEQ ID NO. Ghrelin
BDBM675521 US20240158438, SEQ ID NO. Ghrelin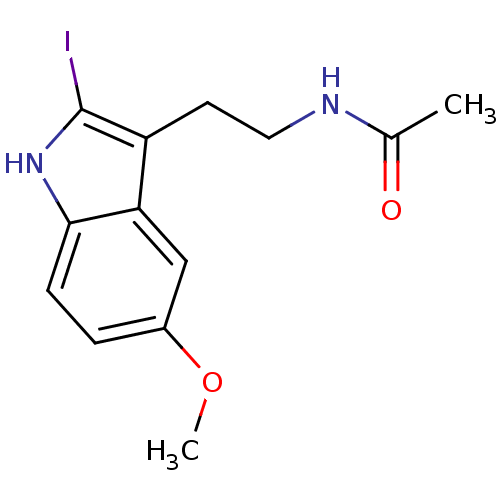 BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo
BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo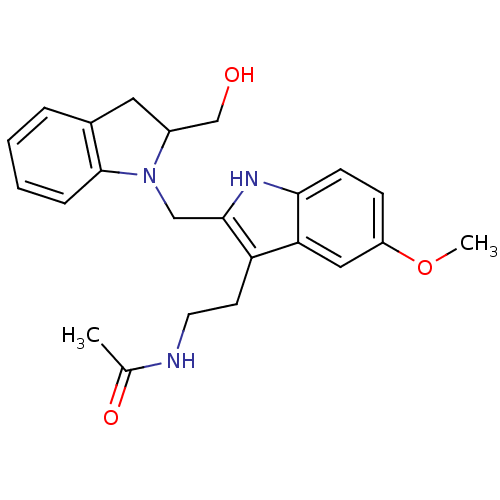 CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin
CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me
BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622
CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622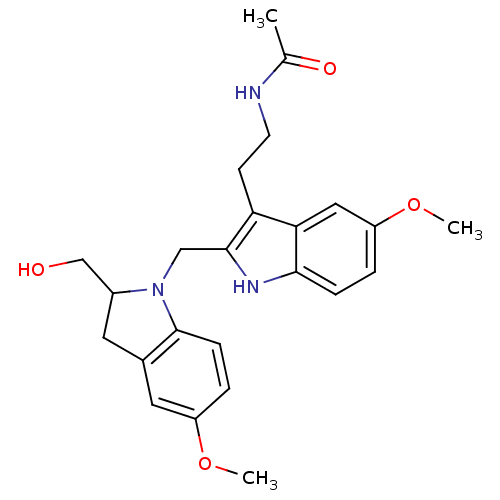 BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin
BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4
BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4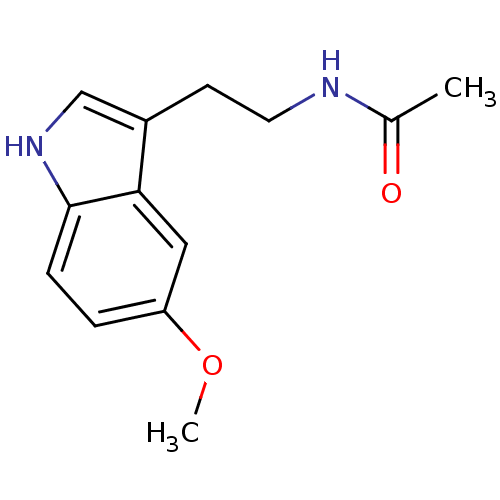 BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide
BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide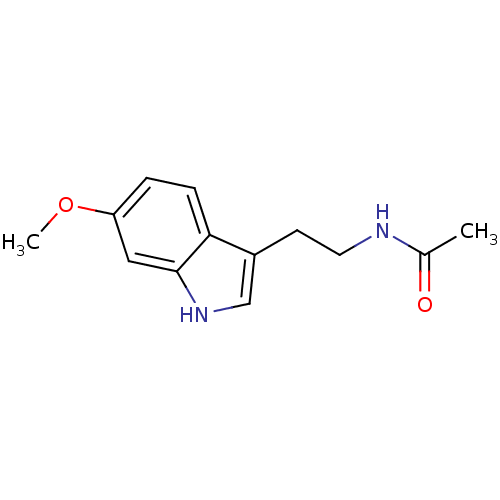 BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy
BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy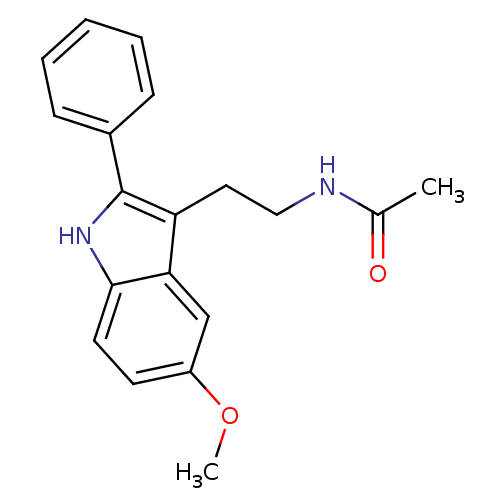 Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110
Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110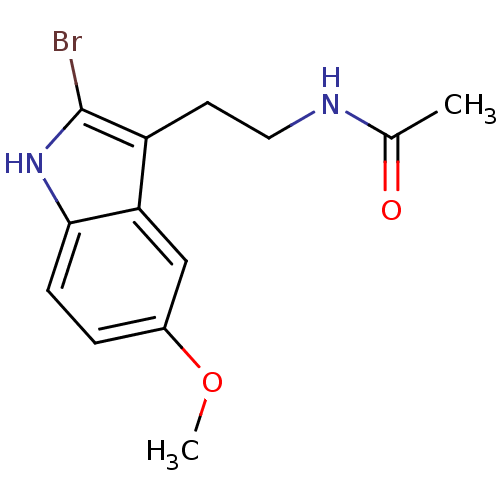 CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin)
CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin) N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730
N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a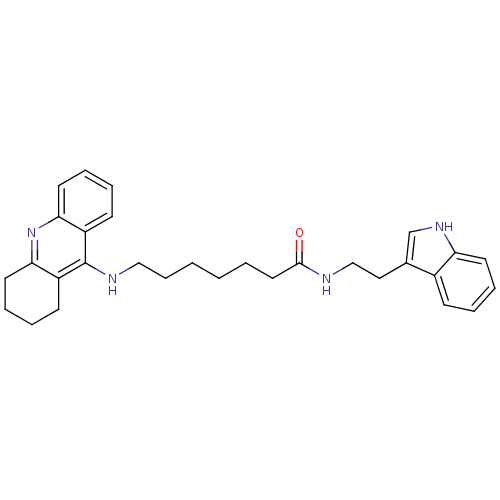 BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide
BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide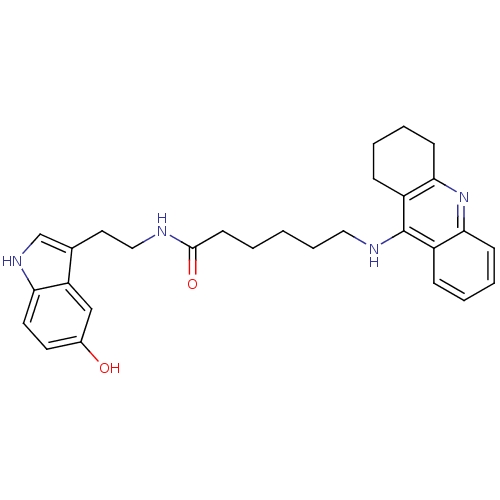 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide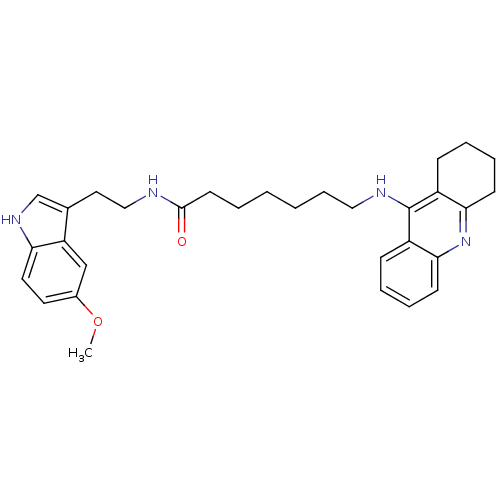 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014
N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014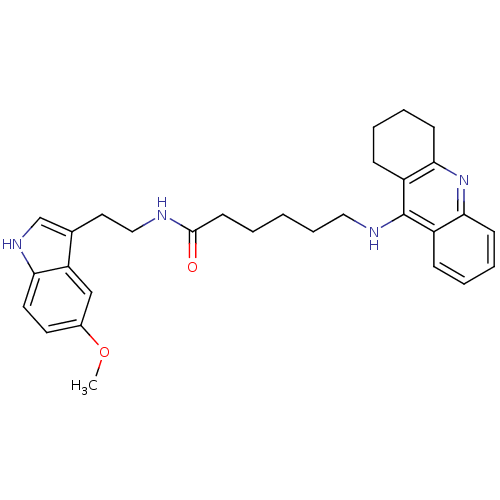 Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide
Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide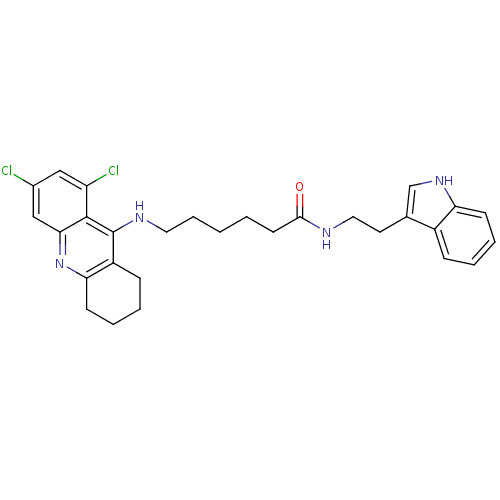 6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a
6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a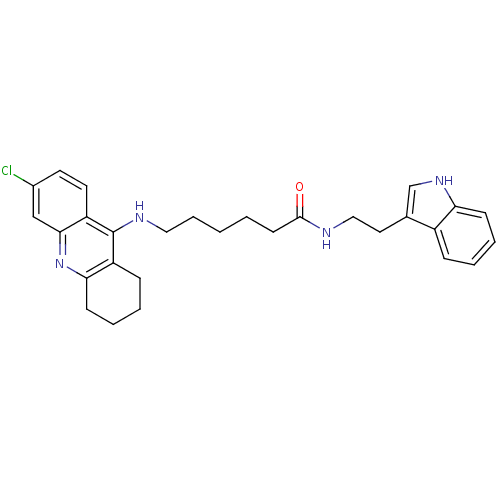 BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a
BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a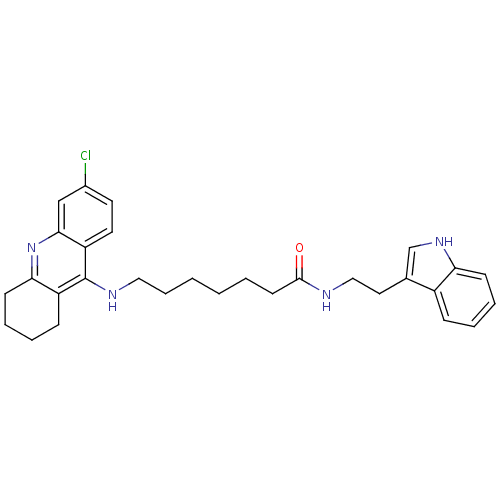 BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide
BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide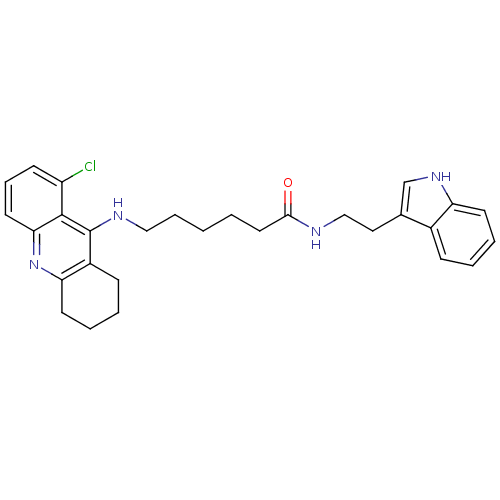 Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010
Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010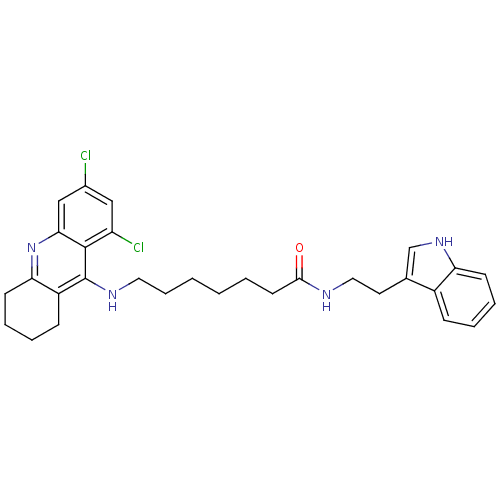 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide
7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide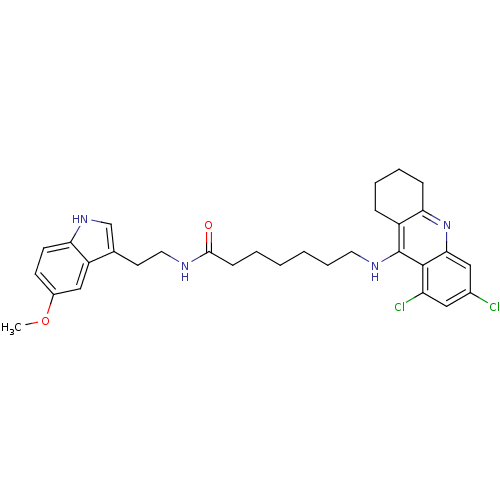 BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide
BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide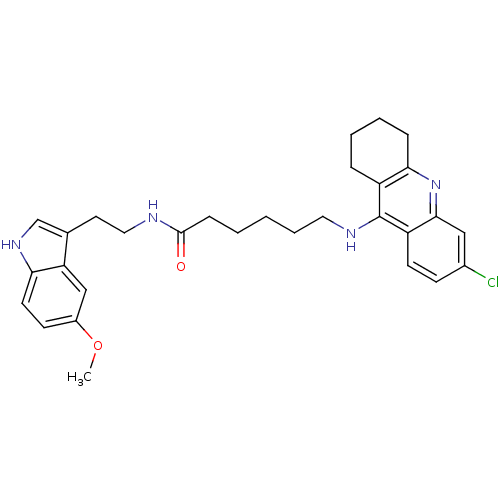 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a
6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a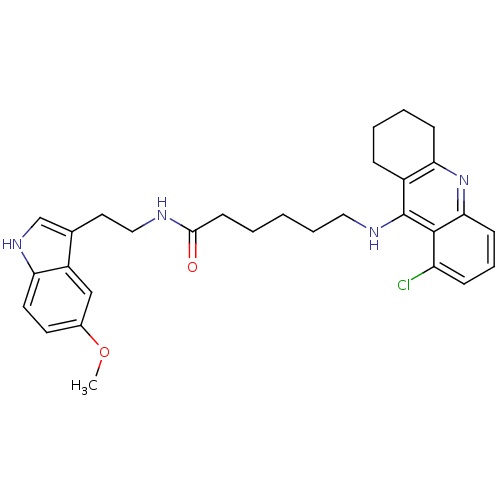 BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide
BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide
- Bandyopadhyay, A; Cheung, M; Eidam, HS; Joshi, H; Su, D Ghrelin O-acyltransferase inhibitors US Patent US11312709 (2022)
- Galka, CS; Hembre, EJ; Honigschmidt, NA; Martinez-Grau, MA; Plaza, GR; Rubio, A; Keding, SJ; Smith, DL Ghrelin 0-acyl transferase inhibitors US Patent US10227310 (2019)
- Galka, CS; Hembre, EJ; Honigschmidt, NA; Martinez-Grau, MA; Plaza, GR; Rubio, A Ghrelin O-acyl transferase inhibitors US Patent US10093651 (2018)
- Sugden, D; Pickering, H; Teh, MT; Garratt, PJ Melatonin receptor pharmacology: toward subtype specificity. Biol Cell 89: 531-7 (1997)
- Mor, M; Rivara, S; Silva, C; Bordi, F; Plazzi, PV; Spadoni, G; Diamantini, G; Balsamini, C; Tarzia, G; Fraschini, F; Lucini, V; Nonno, R; Stankov, BM Melatonin receptor ligands: synthesis of new melatonin derivatives and comprehensive comparative molecular field analysis (CoMFA) study. J Med Chem 41: 3831-44 (1998)
- Iyer, MR; Wood, CM; Kunos, G Recent progress in the discovery of ghrelin RSC Med Chem 11: 1136-1144 (2020)
- Garratt, PJ; Jones, R; Tocher, DA; Sugden, D Mapping the melatonin receptor. 3. Design and synthesis of melatonin agonists and antagonists derived from 2-phenyltryptamines. J Med Chem 38: 1132-9 (1995)
- Reppert, SM; Godson, C; Mahle, CD; Weaver, DR; Slaugenhaupt, SA; Gusella, JF Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A 92: 8734-8 (1995)
- Karageorge, GN; Bertenshaw, S; Iben, L; Xu, C; Sarbin, N; Gentile, A; Dubowchik, GM Tetrahydroisoquinoline derivatives as melatonin MT2 receptor antagonists. Bioorg Med Chem Lett 14: 5881-4 (2004)
- Blayo, AL; Maingot, M; Aicher, B; M'Kadmi, C; Schmidt, P; Müller, G; Teifel, M; Günther, E; Gagne, D; Denoyelle, S; Martinez, J; Fehrentz, JA New trisubstituted 1,2,4-triazoles as ghrelin receptor antagonists. Bioorg Med Chem Lett 25: 20-4 (2014)
- Cameron, KO; Bhattacharya, SK; Loomis, AK Small molecule ghrelin receptor inverse agonists and antagonists. J Med Chem 57: 8671-91 (2014)
- Bukhari, SNA An insight into the multifunctional role of ghrelin and structure activity relationship studies of ghrelin receptor ligands with clinical trials. Eur J Med Chem 235: (2022)
- Zlotos, DP; Attia, MI; Julius, J; Sethi, S; Witt-Enderby, PA 2-[(2,3-dihydro-1H-indol-1-yl)methyl]melatonin analogues: a novel class of MT2-selective melatonin receptor antagonists. J Med Chem 52: 826-33 (2009)
- Davies, DJ; Garratt, PJ; Tocher, DA; Vonhoff, S; Davies, J; Teh, MT; Sugden, D Mapping the melatonin receptor. 5. Melatonin agonists and antagonists derived from tetrahydrocyclopent[b]indoles, tetrahydrocarbazoles and hexahydrocyclohept[b]indoles. J Med Chem 41: 451-67 (1998)
- McGovern-Gooch, KR; Mahajani, NS; Garagozzo, A; Schramm, AJ; Hannah, LG; Sieburg, MA; Chisholm, JD; Hougland, JL Synthetic Triterpenoid Inhibition of Human Ghrelin O-Acyltransferase: The Involvement of a Functionally Required Cysteine Provides Mechanistic Insight into Ghrelin Acylation. Biochemistry 56: 919-931 (2017)
- Morgan, PJ; Barrett, P; Howell, HE; Helliwell, R Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int 24: 101-46 (1994)
- Li, PK; Chu, GH; Gillen, ML; Parekh, T; Witt-Enderby, PA The development of a charged melatonin receptor ligand Bioorg Med Chem Lett 7: 2409-2414 (1997)
- Spadoni, G; Balsamini, C; Diamantini, G; Di Giacomo, B; Tarzia, G; Mor, M; Plazzi, PV; Rivara, S; Lucini, V; Nonno, R; Pannacci, M; Fraschini, F; Stankov, BM Conformationally restrained melatonin analogues: synthesis, binding affinity for the melatonin receptor, evaluation of the biological activity, and molecular modeling study. J Med Chem 40: 1990-2002 (1997)
- Mihalic, JT; Kim, YJ; Lizarzaburu, M; Chen, X; Deignan, J; Wanska, M; Yu, M; Fu, J; Chen, X; Zhang, A; Connors, R; Liang, L; Lindstrom, M; Ma, J; Tang, L; Dai, K; Li, L Discovery of a new class of ghrelin receptor antagonists. Bioorg Med Chem Lett 22: 2046-51 (2012)
- Epperson, JR; Deskus, JA; Gentile, AJ; Iben, LG; Ryan, E; Sarbin, NS 4-Substituted anilides as selective melatonin MT2 receptor agonists. Bioorg Med Chem Lett 14: 1023-6 (2004)
- Beresford, IJ; Browning, C; Starkey, SJ; Brown, J; Foord, SM; Coughlan, J; North, PC; Dubocovich, ML; Hagan, RM GR196429: a nonindolic agonist at high-affinity melatonin receptors. J Pharmacol Exp Ther 285: 1239-45 (1998)
- Wang, SY; Shi, XC; Laborda, P Indole-based melatonin analogues: Synthetic approaches and biological activity. Eur J Med Chem 185: (2020)
- Di Giacomo, B; Bedini, A; Spadoni, G; Tarzia, G; Fraschini, F; Pannacci, M; Lucini, V Synthesis and biological activity of new melatonin dimeric derivatives. Bioorg Med Chem 15: 4643-50 (2007)
- Palucki, BL; Feighner, SD; Pong, S; McKee, KK; Hreniuk, DL; Tan, C; Howard, AD; Van der Ploeg, LH; Patchett, AA; Nargund, RP Spiro(indoline-3,4'-piperidine) growth hormone secretagogues as ghrelin mimetics. Bioorg Med Chem Lett 11: 1955-7 (2001)
- Bednarek, MA; Feighner, SD; Pong, SS; McKee, KK; Hreniuk, DL; Silva, MV; Warren, VA; Howard, AD; Van Der Ploeg, LH; Heck, JV Structure-function studies on the new growth hormone-releasing peptide, ghrelin: minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43: 4370-6 (2000)
- Hoashi, Y; Takai, T; Kosugi, Y; Nakashima, M; Nakayama, M; Hirai, K; Uchikawa, O; Koike, T Discovery of a Potent and Orally Bioavailable Melatonin Receptor Agonist. J Med Chem 64: 3059-3074 (2021)
- Giorgioni, G; Del Bello, F; Quaglia, W; Botticelli, L; Cifani, C; Micioni Di Bonaventura, E; Micioni Di Bonaventura, MV; Piergentili, A Advances in the Development of Nonpeptide Small Molecules Targeting Ghrelin Receptor. J Med Chem 65: 3098-3118 (2022)
- Kung, DW; Coffey, SB; Jones, RM; Cabral, S; Jiao, W; Fichtner, M; Carpino, PA; Rose, CR; Hank, RF; Lopaze, MG; Swartz, R; Chen, HT; Hendsch, Z; Posner, B; Wielis, CF; Manning, B; Dubins, J; Stock, IA; Varma, S; Campbell, M; DeBartola, D; Kosa-Maines, R; Steyn, SJ; McClure, KF Identification of spirocyclic piperidine-azetidine inverse agonists of the ghrelin receptor. Bioorg Med Chem Lett 22: 4281-7 (2012)
- Godbout, C; Trieselmann, T; Vintonyak, V Oxadiazolopyridine derivatives for use as ghrelin O-acyl transferase (GOAT) inhibitors US Patent US10308667 (2019)
- Trieselmann, T; Godbout, C; Hoenke, C; Vintonyak, V Triazolopyrimidine derivatives for use as ghrelin o-acyl transferase (GOAT) inhibitors US Patent US11583532 (2023)
- Takahashi, B; Funami, H; Iwaki, T; Maruoka, H; Nagahira, A; Koyama, M; Kamiide, Y; Matsuo, T; Muto, T; Annoura, H 2-Aminoalkyl nicotinamide derivatives as pure inverse agonists of the ghrelin receptor. Bioorg Med Chem Lett 25: 2707-12 (2015)
- McClure, KF; Jackson, M; Cameron, KO; Kung, DW; Perry, DA; Orr, ST; Zhang, Y; Kohrt, J; Tu, M; Gao, H; Fernando, D; Jones, R; Erasga, N; Wang, G; Polivkova, J; Jiao, W; Swartz, R; Ueno, H; Bhattacharya, SK; Stock, IA; Varma, S; Bagdasarian, V; Perez, S; Kelly-Sullivan, D; Wang, R; Kong, J; Cornelius, P; Michael, L; Lee, E; Janssen, A; Steyn, SJ; Lapham, K; Goosen, T Identification of potent, selective, CNS-targeted inverse agonists of the ghrelin receptor. Bioorg Med Chem Lett 23: 5410-4 (2013)
- Puleo, L; Marini, P; Avallone, R; Zanchet, M; Bandiera, S; Baroni, M; Croci, T Synthesis and pharmacological evaluation of indolinone derivatives as novel ghrelin receptor antagonists. Bioorg Med Chem 20: 5623-36 (2012)
- Copinga, S; Tepper, PG; Grol, CJ; Horn, AS; Dubocovich, ML 2-Amido-8-methoxytetralins: a series of nonindolic melatonin-like agents. J Med Chem 36: 2891-8 (1993)
- Zlotos, DP; Jockers, R; Cecon, E; Rivara, S; Witt-Enderby, PA MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J Med Chem 57: 3161-85 (2014)
- de la Fuente Revenga, M; Herrera-Arozamena, C; Fernández-Sáez, N; Barco, G; García-Orue, I; Sugden, D; Rivara, S; Rodríguez-Franco, MI New coumarin-based fluorescent melatonin ligands. Design, synthesis and pharmacological characterization. Eur J Med Chem 103: 370-3 (2015)
- Thireau, J; Marteaux, J; Delagrange, P; Lefoulon, F; Dufourny, L; Guillaumet, G; Suzenet, F Original Design of Fluorescent Ligands by Fusing BODIPY and Melatonin Neurohormone. ACS Med Chem Lett 5: 158-61 (2014)
- Witherington, J; Abberley, L; Bellenie, BR; Boatman, R; Collis, K; Dean, DK; Gaiba, A; King, NP; Shuker, N; Steadman, JG; Takle, AK; Sanger, G; Butler, S; McKay, F; Muir, A; Winborn, K; Ward, RW; Heightman, TD Aryl sulphonyl amides as potent agonists of the growth hormone secretagogue (ghrelin) receptor. Bioorg Med Chem Lett 19: 684-7 (2009)
- Cooper, M; Llinas, A; Hansen, P; Caffrey, M; Ray, A; Sjödin, S; Shamovsky, I; Wada, H; Jellesmark Jensen, T; Sivars, U; Hultin, L; Andersson, U; Lundqvist, S; Gedda, K; Jinton, L; Krutrök, N; Lewis, R; Jansson, P; Gardelli, C Identification and Optimization of Pyrrolidine Derivatives as Highly Potent Ghrelin Receptor Full Agonists. J Med Chem 63: 9705-9730 (2020)
- Mari, M; Elisi, GM; Bedini, A; Lucarini, S; Retini, M; Lucini, V; Scaglione, F; Vincenzi, F; Varani, K; Castelli, R; Mor, M; Rivara, S; Spadoni, G 2-Arylmelatonin analogues: Probing the 2-phenyl binding pocket of melatonin MT Eur J Med Chem 243: (2022)
- He, F; Chou, CJ; Scheiner, M; Poeta, E; Yuan Chen, N; Gunesch, S; Hoffmann, M; Sotriffer, C; Monti, B; Maurice, T; Decker, M Melatonin- and Ferulic Acid-Based HDAC6 Selective Inhibitors Exhibit Pronounced Immunomodulatory Effects J Med Chem 64: 3794-3812 (2021)
- Bolteau, R; Descamps, F; Ettaoussi, M; Caignard, DH; Delagrange, P; Melnyk, P; Yous, S Quinazoline and phthalazine derivatives as novel melatonin receptor ligands analogues of agomelatine. Eur J Med Chem 189: (2020)
- Li, PK; Chu, GH; Gillen, ML; Witt-Enderby, PA Synthesis and receptor binding studies of quinolinic derivatives as melatonin receptor ligands Bioorg Med Chem Lett 7: 2177-2180 (1997)
- Fukatsu, K; Uchikawa, O; Kawada, M; Yamano, T; Yamashita, M; Kato, K; Hirai, K; Hinuma, S; Miyamoto, M; Ohkawa, S Synthesis of a novel series of benzocycloalkene derivatives as melatonin receptor agonists. J Med Chem 45: 4212-21 (2002)
- Kloubert, S; Mathé-Allainmat, M; Andrieux, J; Sicsic, S; Langlois, M Synthesis of benzocycloalkane derivatives as new conformationally restricted ligands for melatonin receptors. Bioorg Med Chem Lett 8: 3325-30 (1999)
- Rivara, S; Scalvini, L; Lodola, A; Mor, M; Caignard, DH; Delagrange, P; Collina, S; Lucini, V; Scaglione, F; Furiassi, L; Mari, M; Lucarini, S; Bedini, A; Spadoni, G Tetrahydroquinoline Ring as a Versatile Bioisostere of Tetralin for Melatonin Receptor Ligands. J Med Chem 61: 3726-3737 (2018)
- Lucini, V; Pannacci, M; Scaglione, F; Fraschini, F; Rivara, S; Mor, M; Bordi, F; Plazzi, PV; Spadoni, G; Bedini, A; Piersanti, G; Diamantini, G; Tarzia, G Tricyclic alkylamides as melatonin receptor ligands with antagonist or inverse agonist activity. J Med Chem 47: 4202-12 (2004)
- Zhao, F; Darling, JE; Gibbs, RA; Hougland, JL A new class of ghrelin O-acyltransferase inhibitors incorporating triazole-linked lipid mimetic groups. Bioorg Med Chem Lett 25: 2800-3 (2015)
- Esteban, JJ; Mason, JR; Kaminski, J; Ramachandran, R; Luyt, LG A survey of stapling methods to increase affinity, activity, and stability of ghrelin analogues. RSC Med Chem 15: 254-266 (2024)
- Li, HZ; Shao, XX; Shou, LL; Li, N; Liu, YL; Xu, ZG; Guo, ZY Development of Esterase-Resistant and Highly Active Ghrelin Analogs via Thiol-Ene Click Chemistry. ACS Med Chem Lett 13: 1655-1662 (2022)
- Gardelli, C; Wada, H; Ray, A; Caffrey, M; Llinas, A; Shamovsky, I; Tholander, J; Larsson, J; Sivars, U; Hultin, L; Andersson, U; Sanganee, HJ; Stenvall, K; Leidvik, B; Gedda, K; Jinton, L; Rydén Landergren, M; Karabelas, K Identification and Pharmacological Profile of an Indane Based Series of Ghrelin Receptor Full Agonists. J Med Chem 61: 5974-5987 (2018)
- Takahashi, B; Funami, H; Iwaki, T; Maruoka, H; Shibata, M; Koyama, M; Nagahira, A; Kamiide, Y; Kanki, S; Igawa, Y; Muto, T Orally active ghrelin receptor inverse agonists and their actions on a rat obesity model. Bioorg Med Chem 23: 4792-803 (2015)
- Heightman, TD; Scott, JS; Longley, M; Bordas, V; Dean, DK; Elliott, R; Hutley, G; Witherington, J; Abberley, L; Passingham, B; Berlanga, M; de Los Frailes, M; Wise, A; Powney, B; Muir, A; McKay, F; Butler, S; Winborn, K; Gardner, C; Darton, J; Campbell, C; Sanger, G Potent achiral agonists of the ghrelin (growth hormone secretagogue) receptor. Part I: Lead identification. Bioorg Med Chem Lett 17: 6584-7 (2007)
- Witherington, J; Abberley, L; Briggs, MA; Collis, K; Dean, DK; Gaiba, A; King, NP; Kraus, H; Shuker, N; Steadman, JG; Takle, AK; Sanger, G; Wadsworth, G; Butler, S; McKay, F; Muir, A; Winborn, K; Heightman, TD Potent achiral agonists of the growth hormone secretagogue (ghrelin) receptor. Part 2: Lead optimisation. Bioorg Med Chem Lett 18: 2203-5 (2008)
- Witt-Enderby, PA; Chu, GH; Gillen, ML; Li, PK Development of a high-affinity ligand that binds irreversibly to Mel1b melatonin receptors. J Med Chem 40: 4195-8 (1998)
- Sun, LQ; Chen, J; Bruce, M; Deskus, JA; Epperson, JR; Takaki, K; Johnson, G; Iben, L; Mahle, CD; Ryan, E; Xu, C Synthesis and structure-activity relationship of novel benzoxazole derivatives as melatonin receptor agonists. Bioorg Med Chem Lett 14: 3799-802 (2004)
- Uchikawa, O; Fukatsu, K; Tokunoh, R; Kawada, M; Matsumoto, K; Imai, Y; Hinuma, S; Kato, K; Nishikawa, H; Hirai, K; Miyamoto, M; Ohkawa, S Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem 45: 4222-39 (2002)
- The location, physiology, pathology of hippocampus Melatonin MT2 receptor and MT2-selective modulators.
- Faust, R; Garratt, PJ; Jones, R; Yeh, LK; Tsotinis, A; Panoussopoulou, M; Calogeropoulou, T; Teh, MT; Sugden, D Mapping the melatonin receptor. 6. Melatonin agonists and antagonists derived from 6H-isoindolo[2,1-a]indoles, 5,6-dihydroindolo[2,1-a]isoquinolines, and 6,7-dihydro-5H-benzo[c]azepino[2,1-a]indoles. J Med Chem 43: 1050-61 (2000)
- Orr, ST; Beveridge, R; Bhattacharya, SK; Cameron, KO; Coffey, S; Fernando, D; Hepworth, D; Jackson, MV; Khot, V; Kosa, R; Lapham, K; Loria, PM; McClure, KF; Patel, J; Rose, C; Saenz, J; Stock, IA; Storer, G; von Volkenburg, M; Vrieze, D; Wang, G; Xiao, J; Zhang, Y Evaluation and synthesis of polar aryl- and heteroaryl spiroazetidine-piperidine acetamides as ghrelin inverse agonists. ACS Med Chem Lett 6: 156-61 (2015)
- Trieselmann, T; Godbout, C; Vintonyak, V Pyrazole- and indazole-substituted oxadiazolopyridine derivatives for use as ghrelin O-acyl transferase (GOAT) inhibitors US Patent US11136337 (2021)
- Rudolph, J; Esler, WP; O'connor, S; Coish, PD; Wickens, PL; Brands, M; Bierer, DE; Bloomquist, BT; Bondar, G; Chen, L; Chuang, CY; Claus, TH; Fathi, Z; Fu, W; Khire, UR; Kristie, JA; Liu, XG; Lowe, DB; McClure, AC; Michels, M; Ortiz, AA; Ramsden, PD; Schoenleber, RW; Shelekhin, TE; Vakalopoulos, A; Tang, W; Wang, L; Yi, L; Gardell, SJ; Livingston, JN; Sweet, LJ; Bullock, WH Quinazolinone derivatives as orally available ghrelin receptor antagonists for the treatment of diabetes and obesity. J Med Chem 50: 5202-16 (2007)
- Tarzia, G; Diamantini, G; Di Giacomo, B; Spadoni, G; Esposti, D; Nonno, R; Lucini, V; Pannacci, M; Fraschini, F; Stankov, BM 1-(2-Alkanamidoethyl)-6-methoxyindole derivatives: a new class of potent indole melatonin analogues. J Med Chem 40: 2003-10 (1997)
- Reppert, SM; Weaver, DR; Ebisawa, T Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13: 1177-85 (1994)
- Somalo-Barranco, G; Serra, C; Lyons, D; Piggins, HD; Jockers, R; Llebaria, A Design and Validation of the First Family of Photo-Activatable Ligands for Melatonin Receptors. J Med Chem 65: 11229-11240 (2022)
- Nonno, R; Lucini, V; Pannacci, M; Mazzucchelli, C; Angeloni, D; Fraschini, F; Stankov, BM Pharmacological characterization of the human melatonin Mel1a receptor following stable transfection into NIH3T3 cells. Br J Pharmacol 124: 485-92 (1998)
- Chen, SY; Geng, CA; Ma, YB; Huang, XY; Yang, XT; Su, LH; He, XF; Li, TZ; Deng, ZT; Gao, Z; Zhang, XM; Chen, JJ Polybenzyls from Gastrodia elata, their agonistic effects on melatonin receptors and structure-activity relationships. Bioorg Med Chem 27: 3299-3306 (2019)
- Jellimann, C; Mathé-Allainmat, M; Andrieux, J; Kloubert, S; Boutin, JA; Nicolas, JP; Bennejean, C; Delagrange, P; Langlois, M Synthesis of phenalene and acenaphthene derivatives as new conformationally restricted ligands for melatonin receptors. J Med Chem 43: 4051-62 (2000)
- Els, S; Schild, E; Petersen, PS; Kilian, TM; Mokrosinski, J; Frimurer, TM; Chollet, C; Schwartz, TW; Holst, B; Beck-Sickinger, AG An aromatic region to induce a switch between agonism and inverse agonism at the ghrelin receptor. J Med Chem 55: 7437-49 (2012)
- Haj Salah, KB; Haj Salah, KB; Maingot, M; Maingot, M; Blayo, AL; Blayo, AL; M''Kadmi, C; M''Kadmi, C; Damian, M; Damian, M; Mary, S; Mary, S; Cantel, S; Cantel, S; Neasta, J; Neasta, J; Oiry, C; Oiry, C; Péraldi-Roux, S; Péraldi-Roux, S; Fernandez, G; Fernandez, G; Romero, GG; Romero, GG; Perello, M; Perello, M; Marie, J; Marie, J; Banères, JL; Banères, JL; Fehrentz, JA; Fehrentz, JA; Denoyelle, S; Denoyelle, S Development of Nonpeptidic Inverse Agonists of the Ghrelin Receptor (GHSR) Based on the 1,2,4-Triazole Scaffold. J Med Chem 63: 10796-10815 (2020)
- Bhattacharya, SK; Andrews, K; Beveridge, R; Cameron, KO; Chen, C; Dunn, M; Fernando, D; Gao, H; Hepworth, D; Jackson, VM; Khot, V; Kong, J; Kosa, RE; Lapham, K; Loria, PM; Londregan, AT; McClure, KF; Orr, ST; Patel, J; Rose, C; Saenz, J; Stock, IA; Storer, G; VanVolkenburg, M; Vrieze, D; Wang, G; Xiao, J; Zhang, Y Discovery of PF-5190457, a Potent, Selective, and Orally Bioavailable Ghrelin Receptor Inverse Agonist Clinical Candidate. ACS Med Chem Lett 5: 474-9 (2014)
- Rosita, D; Dewit, MA; Luyt, LG Fluorine and rhenium substituted ghrelin analogues as potential imaging probes for the growth hormone secretagogue receptor. J Med Chem 52: 2196-203 (2009)
- Pedretti, A; Vistoli, G Modeling of human ghrelin receptor (hGHS-R1a) in its close state and validation by molecular docking. Bioorg Med Chem 15: 3054-64 (2007)
- Ettaoussi, M; Péres, B; Klupsch, F; Delagrange, P; Boutin, JA; Renard, P; Caignard, DH; Chavatte, P; Berthelot, P; Lesieur, D; Yous, S Design and synthesis of benzofuranic derivatives as new ligands at the melatonin-binding site MT3. Bioorg Med Chem 16: 4954-62 (2008)
- Leclerc, V; Ettaoussi, M; Rami, M; Farce, A; Boutin, JA; Delagrange, P; Caignard, DH; Renard, P; Berthelot, P; Yous, S Design and synthesis of naphthalenic derivatives as new ligands at the melatonin binding site MT3. Eur J Med Chem 46: 1622-9 (2011)
- Hasan, M; Genovese, S; Fiorito, S; Epifano, F; Witt-Enderby, PA Oxyprenylated Phenylpropanoids Bind to MT1 Melatonin Receptors and Inhibit Breast Cancer Cell Proliferation and Migration. J Nat Prod 80: 3324-3329 (2017)
- Pasternak, A; Goble, SD; deJesus, RK; Hreniuk, DL; Chung, CC; Tota, MR; Mazur, P; Feighner, SD; Howard, AD; Mills, SG; Yang, L Discovery and optimization of novel 4-[(aminocarbonyl)amino]-N-[4-(2-aminoethyl)phenyl]benzenesulfonamide ghrelin receptor antagonists. Bioorg Med Chem Lett 19: 6237-40 (2009)
- M'Kadmi, C; Cabral, A; Barrile, F; Giribaldi, J; Cantel, S; Damian, M; Mary, S; Denoyelle, S; Dutertre, S; Péraldi-Roux, S; Neasta, J; Oiry, C; Banères, JL; Marie, J; Perello, M; Fehrentz, JA N-Terminal Liver-Expressed Antimicrobial Peptide 2 (LEAP2) Region Exhibits Inverse Agonist Activity toward the Ghrelin Receptor. J Med Chem 62: 965-973 (2019)
- Kilian, TM; Klöting, N; Bergmann, R; Els-Heindl, S; Babilon, S; Clément-Ziza, M; Zhang, Y; Beck-Sickinger, AG; Chollet, C Rational design of dual peptides targeting ghrelin and Y2 receptors to regulate food intake and body weight. J Med Chem 58: 4180-93 (2015)
- Aicher, B; Mueller, G; Paulini, K; Blumenstein, L; Schmidt, P; Gerlach, M; Teifel, M; Martinez, J; Fehrentz, J; Blayo, A Triazole derivatives with improved receptor activity and bioavailability properties as ghrelin antagonists of growth hormone secretagogue receptors US Patent US8546435 (2013)
- Spadoni, G; Balsamini, C; Diamantini, G; Tontini, A; Tarzia, G; Mor, M; Rivara, S; Plazzi, PV; Nonno, R; Lucini, V; Pannacci, M; Fraschini, F; Stankov, BM 2-N-acylaminoalkylindoles: design and quantitative structure-activity relationship studies leading to MT2-selective melatonin antagonists. J Med Chem 44: 2900-12 (2001)
- Du, H; Wang, J; Zhang, X; Hu, Z A novel quantitative structure-activity relationship method to predict the affinities of MT3 melatonin binding site. Eur J Med Chem 43: 2861-9 (2008)
- Rivara, S; Lorenzi, S; Mor, M; Plazzi, PV; Spadoni, G; Bedini, A; Tarzia, G Analysis of structure-activity relationships for MT2 selective antagonists by melatonin MT1 and MT2 receptor models. J Med Chem 48: 4049-60 (2005)
- Castro-Palomino Laria, JC; Camacho Gómez, JA; Mendoza Lizaldez, A Derivatives of 2-aminopyridine as adenosine A2B receptor antagonists and ligands of the melatonin MT3 receptors US Patent US10253017 (2019)
- Jeanty, M; Suzenet, F; Delagrange, P; Nosjean, O; Boutin, JA; Caignard, DH; Guillaumet, G Design and synthesis of 1-(2-alkanamidoethyl)-6-methoxy-7-azaindole derivatives as potent melatonin agonists. Bioorg Med Chem Lett 21: 2316-9 (2011)
- Sun, LQ; Takaki, K; Chen, J; Iben, L; Knipe, JO; Pajor, L; Mahle, CD; Ryan, E; Xu, C N-[2-[2-(4-Phenylbutyl)benzofuran-4-yl]cyclopropylmethyl]acetamide: an orally bioavailable melatonin receptor agonist. Bioorg Med Chem Lett 14: 5157-60 (2004)
- Marot, C; Chavatte, P; Morin-Allory, L; Viaud, MC; Guillaumet, G; Renard, P; Lesieur, D; Michel, A Pharmacophoric search and 3D-QSAR comparative molecular field analysis studies on agonists of melatonin sheep receptors. J Med Chem 41: 4453-65 (1998)
- Li, TZ; Hu, J; Sun, JJ; Huang, XY; Geng, CA; Liu, SB; Zhang, XM; Chen, JJ Synthesis and biological evaluation of paeoveitol D derivatives as new melatonin receptor agonists with antidepressant activities. RSC Med Chem 13: 1212-1224 (2022)
- Sicsic, S; Serraz, I; Andrieux, J; Brémont, B; Mathé-Allainmat, M; Poncet, A; Shen, S; Langlois, M Three-dimensional quantitative structure-activity relationship of melatonin receptor ligands: a comparative molecular field analysis study. J Med Chem 40: 739-48 (1997)
- Pedretti, A; Villa, M; Pallavicini, M; Valoti, E; Vistoli, G Construction of human ghrelin receptor (hGHS-R1a) model using a fragmental prediction approach and validation through docking analysis. J Med Chem 49: 3077-85 (2006)
- Holst, B; Mokrosinski, J; Lang, M; Brandt, E; Nygaard, R; Frimurer, TM; Beck-Sickinger, AG; Schwartz, TW Identification of an efficacy switch region in the ghrelin receptor responsible for interchange between agonism and inverse agonism. J Biol Chem 282: 15799-811 (2007)
- Faust, R; Garratt, PJ; Trujillo Pérez, MA; Piccio, VJ; Madsen, C; Stenstrøm, A; Frølund, B; Davidson, K; Teh, MT; Sugden, D 7-Substituted-melatonin and 7-substituted-1-methylmelatonin analogues: effect of substituents on potency and binding affinity. Bioorg Med Chem 15: 4543-51 (2007)
- El Kazzouli, S; Griffon du Bellay, A; Berteina-Raboin, S; Delagrange, P; Caignard, DH; Guillaumet, G Design and synthesis of 2-phenylimidazo[1,2-a]pyridines as a novel class of melatonin receptor ligands. Eur J Med Chem 46: 4252-7 (2011)
- Carocci, A; Catalano, A; Lovece, A; Lentini, G; Duranti, A; Lucini, V; Pannacci, M; Scaglione, F; Franchini, C Design, synthesis, and pharmacological effects of structurally simple ligands for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem 18: 6496-511 (2010)
- Garratt, PJ; Travard, S; Vonhoff, S; Tsotinis, A; Sugden, D Mapping the melatonin receptor. 4. Comparison of the binding affinities of a series of substituted phenylalkyl amides. J Med Chem 39: 1797-805 (1996)
- Rivara, S; Lodola, A; Mor, M; Bedini, A; Spadoni, G; Lucini, V; Pannacci, M; Fraschini, F; Scaglione, F; Sanchez, RO; Gobbi, G; Tarzia, G N-(substituted-anilinoethyl)amides: design, synthesis, and pharmacological characterization of a new class of melatonin receptor ligands. J Med Chem 50: 6618-26 (2007)
- Depreux, P; Lesieur, D; Mansour, HA; Morgan, P; Howell, HE; Renard, P; Caignard, DH; Pfeiffer, B; Delagrange, P; Guardiola, B Synthesis and structure-activity relationships of novel naphthalenic and bioisosteric related amidic derivatives as melatonin receptor ligands. J Med Chem 37: 3231-9 (1994)
- Wallez, V; Durieux-Poissonnier, S; Chavatte, P; Boutin, JA; Audinot, V; Nicolas, JP; Bennejean, C; Delagrange, P; Renard, P; Lesieur, D Synthesis and structure-affinity-activity relationships of novel benzofuran derivatives as MT(2) melatonin receptor selective ligands. J Med Chem 45: 2788-800 (2002)
- Mathé-Allainmat, M; Gaudy, F; Sicsic, S; Dangy-Caye, AL; Shen, S; Brémont, B; Benatalah, Z; Langlois, M; Renard, P; Delagrange, P Synthesis of 2-amido-2,3-dihydro-1H-phenalene derivatives as new conformationally restricted ligands for melatonin receptors. J Med Chem 39: 3089-95 (1996)
- Morellato, L; Lefas-Le Gall, M; Langlois, M; Caignard, DH; Renard, P; Delagrange, P; Mathé-Allainmat, M Synthesis of new N-(arylcyclopropyl)acetamides and N-(arylvinyl)acetamides as conformationally-restricted ligands for melatonin receptors. Bioorg Med Chem Lett 23: 430-4 (2012)
- Hu, Y; Ho, MK; Chan, KH; New, DC; Wong, YH Synthesis of substituted N-[3-(3-methoxyphenyl)propyl] amides as highly potent MT(2)-selective melatonin ligands. Bioorg Med Chem Lett 20: 2582-5 (2010)
- Maingot, M; Blayo, AL; Denoyelle, S; M'Kadmi, C; Damian, M; Mary, S; Gagne, D; Sanchez, P; Aicher, B; Schmidt, P; Müller, G; Teifel, M; Günther, E; Marie, J; Banères, JL; Martinez, J; Fehrentz, JA New ligands of the ghrelin receptor based on the 1,2,4-triazole scaffold by introduction of a second chiral center. Bioorg Med Chem Lett 26: 2408-12 (2016)
- Synthesis and biological evaluation of novel 18F-labeled 2,4-diaminopyrimidine derivatives for detection of ghrelin receptor in the brain.
- Spadoni, G; Balsamini, C; Bedini, A; Diamantini, G; Di Giacomo, B; Tontini, A; Tarzia, G; Mor, M; Plazzi, PV; Rivara, S; Nonno, R; Pannacci, M; Lucini, V; Fraschini, F; Stankov, BM 2-[N-Acylamino(C1-C3)alkyl]indoles as MT1 melatonin receptor partial agonists, antagonists, and putative inverse agonists. J Med Chem 41: 3624-34 (1998)
- Hu, Y; Zhu, J; Chan, KH; Wong, YH Development of substituted N-[3-(3-methoxylphenyl)propyl] amides as MT(2)-selective melatonin agonists: improving metabolic stability. Bioorg Med Chem 21: 547-52 (2012)
- Spadoni, G; Bedini, A; Lucarini, S; Mari, M; Caignard, DH; Boutin, JA; Delagrange, P; Lucini, V; Scaglione, F; Lodola, A; Zanardi, F; Pala, D; Mor, M; Rivara, S Highly Potent and Selective MT2 Melatonin Receptor Full Agonists from Conformational Analysis of 1-Benzyl-2-acylaminomethyl-tetrahydroquinolines. J Med Chem 58: 7512-25 (2015)
- Calamini, B; Santarsiero, BD; Boutin, JA; Mesecar, AD Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem J 413: 81-91 (2008)
- Nonno, R; Pannacci, M; Lucini, V; Angeloni, D; Fraschini, F; Stankov, BM Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists. Br J Pharmacol 127: 1288-94 (1999)
- de la Fuente Revenga, M; Fernández-Sáez, N; Herrera-Arozamena, C; Morales-García, JA; Alonso-Gil, S; Pérez-Castillo, A; Caignard, DH; Rivara, S; Rodríguez-Franco, MI Novel N-Acetyl Bioisosteres of Melatonin: Melatonergic Receptor Pharmacology, Physicochemical Studies, and Phenotypic Assessment of Their Neurogenic Potential. J Med Chem 58: 4998-5014 (2015)
- Rodriguez-Franco, MI; Fernandez-Bachiller, MI; Perez, C; Hernandez-Ledesma, B; Bartolome, B Novel tacrine-melatonin hybrids as dual-acting drugs for Alzheimer disease, with improved acetylcholinesterase inhibitory and antioxidant properties. J Med Chem 49: 459-62 (2006)
- Moulin, A; Demange, L; Ryan, J; Mousseaux, D; Sanchez, P; Bergé, G; Gagne, D; Perrissoud, D; Locatelli, V; Torsello, A; Galleyrand, JC; Fehrentz, JA; Martinez, J New trisubstituted 1,2,4-triazole derivatives as potent ghrelin receptor antagonists. 3. Synthesis and pharmacological in vitro and in vivo evaluations. J Med Chem 51: 689-93 (2008)
- Daina, A; Giuliano, C; Pietra, C; Wang, J; Chi, Y; Zou, Z; Li, F; Yan, Z; Zhou, Y; Guainazzi, A; Garcia Rubio, S; Zoete, V Rational Design, Synthesis, and Pharmacological Characterization of Novel Ghrelin Receptor Inverse Agonists as Potential Treatment against Obesity-Related Metabolic Diseases. J Med Chem 61: 11039-11060 (2018)
- Demange, L; Boeglin, D; Moulin, A; Mousseaux, D; Ryan, J; Bergé, G; Gagne, D; Heitz, A; Perrissoud, D; Locatelli, V; Torsello, A; Galleyrand, JC; Fehrentz, JA; Martinez, J Synthesis and pharmacological in vitro and in vivo evaluations of novel triazole derivatives as ligands of the ghrelin receptor. 1. J Med Chem 50: 1939-57 (2007)
- Moulin, A; Demange, L; Ryan, J; M'Kadmi, C; Galleyrand, JC; Martinez, J; Fehrentz, JA Trisubstituted 1,2,4-triazoles as ligands for the ghrelin receptor: on the significance of the orientation and substitution at position 3. Bioorg Med Chem Lett 18: 164-8 (2008)
- Spadoni, G; Stankov, B; Duranti, A; Biella, G; Lucini, V; Salvatori, A; Fraschini, F 2-Substituted 5-methoxy-N-acyltryptamines: synthesis, binding affinity for the melatonin receptor, and evaluation of the biological activity. J Med Chem 36: 4069-74 (1993)
- Luo, XT; Wang, CM; Liu, Y; Huang, ZG New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant, and neuroprotective properties as innovative drugs for Alzheimer's disease. Eur J Med Chem 103: 302-11 (2015)
- Herrera-Arozamena, C; Estrada-Valencia, M; Pérez, C; Lagartera, L; Morales-García, JA; Pérez-Castillo, A; Franco-Gonzalez, JF; Michalska, P; Duarte, P; León, R; López, MG; Mills, A; Gago, F; García-Yagüe, ÁJ; Fernández-Ginés, R; Cuadrado, A; Rodríguez-Franco, MI Tuning melatonin receptor subtype selectivity in oxadiazolone-based analogues: Discovery of QR2 ligands and NRF2 activators with neurogenic properties. Eur J Med Chem 190: (2020)
- Moulin, A; Demange, L; Bergé, G; Gagne, D; Ryan, J; Mousseaux, D; Heitz, A; Perrissoud, D; Locatelli, V; Torsello, A; Galleyrand, JC; Fehrentz, JA; Martinez, J Toward Potent Ghrelin Receptor Ligands Based on Trisubstituted 1,2,4-Triazole Structure. 2. Synthesis and Pharmacological in Vitro and in Vivo Evaluations. J Med Chem 50: 5790-5806 (2007)
- Liu, P; Cheng, M; Guo, J; Cao, D; Luo, J; Wan, Y; Fang, Y; Jin, Y; Xie, SS; Liu, J Dual functional antioxidant and butyrylcholinesterase inhibitors for the treatment of Alzheimer's disease: Design, synthesis and evaluation of novel melatonin-alkylbenzylamine hybrids. Bioorg Med Chem 78: (2023)
- Wang, J; Wang, ZM; Li, XM; Li, F; Wu, JJ; Kong, LY; Wang, XB Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer's disease based on the fusion of donepezil and melatonin. Bioorg Med Chem 24: 4324-4338 (2016)
- Hoveyda, HR; Marsault, E; Gagnon, R; Mathieu, AP; Vézina, M; Landry, A; Wang, Z; Benakli, K; Beaubien, S; Saint-Louis, C; Brassard, M; Pinault, JF; Ouellet, L; Bhat, S; Ramaseshan, M; Peng, X; Foucher, L; Beauchemin, S; Bhérer, P; Veber, DF; Peterson, ML; Fraser, GL Optimization of the potency and pharmacokinetic properties of a macrocyclic ghrelin receptor agonist (Part I): Development of ulimorelin (TZP-101) from hit to clinic. J Med Chem 54: 8305-20 (2011)
- Rivara, S; Mor, M; Silva, C; Zuliani, V; Vacondio, F; Spadoni, G; Bedini, A; Tarzia, G; Lucini, V; Pannacci, M; Fraschini, F; Plazzi, PV Three-dimensional quantitative structure-activity relationship studies on selected MT1 and MT2 melatonin receptor ligands: requirements for subtype selectivity and intrinsic activity modulation. J Med Chem 46: 1429-39 (2003)
- Mary, S; Fehrentz, JA; Damian, M; Gaibelet, G; Orcel, H; Verdié, P; Mouillac, B; Martinez, J; Marie, J; Banères, JL Heterodimerization with Its splice variant blocks the ghrelin receptor 1a in a non-signaling conformation: a study with a purified heterodimer assembled into lipid discs. J Biol Chem 288: 24656-65 (2013)
- Spadoni, G; Bedini, A; Furiassi, L; Mari, M; Mor, M; Scalvini, L; Lodola, A; Ghidini, A; Lucini, V; Dugnani, S; Scaglione, F; Piomelli, D; Jung, KM; Supuran, CT; Lucarini, L; Durante, M; Sgambellone, S; Masini, E; Rivara, S Identification of Bivalent Ligands with Melatonin Receptor Agonist and Fatty Acid Amide Hydrolase (FAAH) Inhibitory Activity That Exhibit Ocular Hypotensive Effect in the Rabbit. J Med Chem 61: 7902-7916 (2018)
- López-Iglesias, B; Pérez, C; Morales-García, JA; Alonso-Gil, S; Pérez-Castillo, A; Romero, A; López, MG; Villarroya, M; Conde, S; Rodríguez-Franco, MI New melatonin-N,N-dibenzyl(N-methyl)amine hybrids: potent neurogenic agents with antioxidant, cholinergic, and neuroprotective properties as innovative drugs for Alzheimer's disease. J Med Chem 57: 3773-85 (2014)
- Spadoni, G; Bedini, A; Orlando, P; Lucarini, S; Tarzia, G; Mor, M; Rivara, S; Lucini, V; Pannacci, M; Scaglione, F Bivalent ligand approach on N-{2-[(3-methoxyphenyl)methylamino]ethyl}acetamide: synthesis, binding affinity and intrinsic activity for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem 19: 4910-6 (2011)
- Tsotinis, A; Vlachou, M; Papahatjis, DP; Calogeropoulou, T; Nikas, SP; Garratt, PJ; Piccio, V; Vonhoff, S; Davidson, K; Teh, MT; Sugden, D Mapping the melatonin receptor. 7. Subtype selective ligands based on beta-substituted N-acyl-5-methoxytryptamines and beta-substituted N-acyl-5-methoxy-1-methyltryptamines. J Med Chem 49: 3509-19 (2006)
- Koike, T; Takai, T; Hoashi, Y; Nakayama, M; Kosugi, Y; Nakashima, M; Yoshikubo, S; Hirai, K; Uchikawa, O Synthesis of a novel series of tricyclic dihydrofuran derivatives: discovery of 8,9-dihydrofuro[3,2-c]pyrazolo[1,5-a]pyridines as melatonin receptor (MT1/MT2) ligands. J Med Chem 54: 4207-18 (2011)
- Millan, MJ; Gobert, A; Lejeune, F; Dekeyne, A; Newman-Tancredi, A; Pasteau, V; Rivet, JM; Cussac, D The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306: 954-64 (2003)
- Teh, MT; Sugden, D Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores. Naunyn Schmiedebergs Arch Pharmacol 358: 522-8 (1998)
- Dubocovich, ML; Masana, MI; Iacob, S; Sauri, DM Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol 355: 365-75 (1997)
- Benchekroun, M; Romero, A; Egea, J; León, R; Michalska, P; Buendía, I; Jimeno, ML; Jun, D; Janockova, J; Sepsova, V; Soukup, O; Bautista-Aguilera, OM; Refouvelet, B; Ouari, O; Marco-Contelles, J; Ismaili, L The Antioxidant Additive Approach for Alzheimer's Disease Therapy: New Ferulic (Lipoic) Acid Plus Melatonin Modified Tacrines as Cholinesterases Inhibitors, Direct Antioxidants, and Nuclear Factor (Erythroid-Derived 2)-Like 2 Activators. J Med Chem 59: 9967-9973 (2016)
- ChEMBL_306659 (CHEMBL831432) Inhibition of [125I]ghrelin binding to human recombinant ghrelin receptor membrane preparation
- ChEMBL_555532 (CHEMBL963792) Inhibition of ghrelin receptor
- ChEMBL_104616 (CHEMBL715564) Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin
- ChEMBL_448111 (CHEMBL898367) Agonist activity at ghrelin receptor
- ChEMBL_449509 (CHEMBL899776) Agonist activity at ghrelin receptor
- ChEMBL_559948 (CHEMBL1013742) Inhibition of human Ghrelin receptor
- ChEMBL_604239 (CHEMBL1050384) Binding affinity to ghrelin receptor
- ChEMBL_879207 (CHEMBL2208520) Agonist activity at ghrelin receptor
- ChEMBL_104930 (CHEMBL714710) Binding affinity against ovine pars tuberalis melatonin receptor using 2-[125I]- melatonin radioligand binding assay
- ChEMBL_879205 (CHEMBL2208518) Antagonist activity at ghrelin receptor assessed as inhibition of ghrelin-induced Ca2+ release by cell based assay
- ChEMBL_104755 (CHEMBL712438) Binding affinity towards melatonin receptor
- ChEMBL_104780 (CHEMBL874168) inhibitory concentration against Melatonin receptor
- ChEMBL_1438516 (CHEMBL3385506) Binding affinity to human ghrelin receptor
- ChEMBL_495127 (CHEMBL1008906) Agonist activity at human Ghrelin receptor
- ChEMBL_622562 (CHEMBL1117506) Agonist activity at human ghrelin receptor
- ChEMBL_1361892 (CHEMBL3293067) Displacement of [125I]ghrelin from human ghrelin receptor expressed in HEK293 cells after 8 hrs by scintillation proximity assay
- ChEMBL_1500304 (CHEMBL3588615) Antagonist activity against ghrelin receptor (unknown origin) expressed in CHO cells assessed as inhibition of ghrelin-induced calcium response
- ChEMBL_1500300 (CHEMBL3588724) Binding affinity to ghrelin receptor (unknown origin)
- ChEMBL_658558 (CHEMBL1247891) Binding affinity to human recombinant Ghrelin receptor
- ChEMBL_834030 (CHEMBL2072770) Displacement of [125I]ghrelin from GHS receptor
- Competitive Binding Assay Competitive binding assay using ghrelin receptor.
- ChEMBL_624959 (CHEMBL1107082) Displacement of [3H]melatonin from human melatonin MT1 receptor expressed in CHO cells after 60 mins by scintillation counting
- ChEMBL_624960 (CHEMBL1107083) Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting
- ChEMBL_742240 (CHEMBL1768465) Inhibition of [125I]Ghrelin binding to human Ghrelin receptor expressed in CHO-K1 cells after 60 mins by liquid scintillation spectrometry
- ChEBML_104949 Binding affinity towards human melatonin receptor type 1A
- ChEBML_105091 In vitro receptor binding at MT1 (Melatonin) receptor.
- ChEBML_105255 In vitro receptor binding at MT2 (Melatonin) receptor.
- ChEBML_105277 Binding affinity towards human melatonin receptor type 1B
- ChEMBL_105242 (CHEMBL712572) Binding affinity against melatonin receptor type 1A
- ChEMBL_305096 (CHEMBL832400) Inhibitory concentration against Melatonin receptor type 1A
- ChEMBL_305097 (CHEMBL832401) Inhibitory concentration against Melatonin receptor type 1B
- ChEMBL_644101 (CHEMBL1212000) Inhibition of human melatonin receptor type 1B
- ChEMBL_811976 (CHEMBL2013346) Displacement of [125I]Ghrelin from human GHSR1a receptor
- ChEMBL_1496677 (CHEMBL3579248) Displacement of [125I]ghrelin from human eYFP-fused ghrelin receptor expressed in COS7 cells after 75 mins by competitive receptor binding assay
- ChEMBL_1505777 (CHEMBL3595015) Displacement of [125I]human ghrelin from rat ghrelin receptor expressed in CHO cell membranes incubated for 30 mins by scintillation counting method
- ChEMBL_1505778 (CHEMBL3595016) Displacement of [125I]human ghrelin from human ghrelin receptor expressed in CHO cell membranes incubated for 30 mins by scintillation counting method
- ChEMBL_862728 (CHEMBL2174353) Displacement of [125I]-His-ghrelin from human ghrelin receptor expressed in COS7 cells incubated for 75 mins by scintillation counting based assay
- ChEMBL_306821 (CHEMBL831012) Ability to displace [125I]ghrelin from cloned human GHS-R expressed in CHO-K cells was determined (Kd of ghrelin is 0.4 nM)
- Binding Assay Binding assay using melatonin receptors 1 or 2.
- ChEMBL_104619 (CHEMBL715567) Binding affinity towards melatonin receptor in chicken brain.
- ChEMBL_105110 (CHEMBL715953) Binding Affinity (pKi) towards Melatonin receptor type 1A
- ChEMBL_105255 (CHEMBL710591) In vitro receptor binding at MT2 (Melatonin) receptor.
- ChEMBL_1452145 (CHEMBL3365091) Antagonist activity against human ghrelin receptor by FLIPR assay
- ChEMBL_879206 (CHEMBL2208519) Agonist activity at ghrelin receptor by cell based assay
- ChEMBL_306726 (CHEMBL831489) Binding affinity to displace [125I]ghrelin from cloned human GHS-R expressed in CHO-K cells was determined (Kd of ghrelin is 0.4 nM)
- ChEMBL_1361893 (CHEMBL3293068) Inverse agonist activity at human ghrelin receptor expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP-gamma-S binding by DELFIA
- ChEMBL_1505782 (CHEMBL3595020) Antagonist activity at rat ghrelin receptor expressed in CHO cells assessed as blocking of human ghrelin-induced response by FLIPR based intracellular Ca2+ mobilization assay
- ChEMBL_1452141 (CHEMBL3365087) Binding affinity to human ghrelin receptor by receptor binding assay
- ChEMBL_1632021 (CHEMBL3874727) Inhibition of ghrelin receptor (unknown origin) by radioligand binding assay
- ChEMBL_449846 (CHEMBL897910) Displacement of [125I]ghrelin from human GHSR1a after 1 hr
- ChEMBL_104752 (CHEMBL712435) Binding affinity towards melatonin receptor in Chicken retinal membranes
- ChEMBL_104939 (CHEMBL714718) Inhibitory concentration against melatonin receptor by nonlinear fiting strategies
- ChEMBL_105111 (CHEMBL715954) Binding Affinity (pKi) towards human Melatonin receptor type 1A
- ChEMBL_105243 (CHEMBL712573) Binding affinity against human Melatonin receptor type 1A (MT1)
- ChEMBL_105272 (CHEMBL858415) Binding Affinity (pKi) towards human Melatonin receptor type 1B
- ChEMBL_105275 (CHEMBL718943) Binding affinity against human Melatonin receptor type 1B (MT2)
- ChEMBL_1505783 (CHEMBL3595021) Antagonist activity at ghrelin receptor in Sprague-Dawley rat primary pituitary cells assessed as inhibition of ghrelin-induced growth hormone secretion incubated for 15 mins by ELISA method
- ChEBML_105102 Binding affinity against human MT1 melatonin receptor expressed in NIH3T3 cells.
- ChEBML_105266 Binding affinity against human MT2 melatonin receptor expressed in NIH3T3 cells
- ChEMBL_104615 (CHEMBL715563) Inhibition of 2-[125I]- iodomelatonin from chicken brain melatonin receptors
- ChEMBL_104620 (CHEMBL715568) Binding affinity towards melatonin receptor in Chicken brain Experiment 1
- ChEMBL_104751 (CHEMBL715876) Binding affinity towards melatonin receptor in Chicken brain Experiment 2
- ChEMBL_104774 (CHEMBL714919) Agonist activity against melatonin receptor in the presence of iodomelatonin
- ChEMBL_104775 (CHEMBL714920) Agonist activity against melatonin receptor in the presence of iodomelatonin
- ChEMBL_1824682 (CHEMBL4324446) Displacement of 2-[125I]iodomelatonin from melatonin receptor (unknown origin)
- ChEMBL_1824703 (CHEMBL4324467) Displacement of 2-[125I]iodomelatonin from chick brain melatonin receptor
- ChEMBL_2238442 (CHEMBL5152338) Binding affinity to human melatonin MT1 assessed as inhibition constant
- ChEBML_72507 Inhibition of ghrelin binding to Growth hormone secretagogue receptor expressed in BHK cells
- ChEMBL_1500296 (CHEMBL3588720) Inverse agonist activity at rat ghrelin receptor by inositol phosphate turnover assay
- ChEMBL_2349020 Displacement of [125I]ghrelin from GHSR1a in HEK293 cells by competitive binding assay
- ChEMBL_460934 (CHEMBL944882) Displacement of 125I]-His9-ghrelin from human GHSR1a expressed in LLCPK1 cells
- ChEMBL_515971 (CHEMBL994776) Agonist activity at human ghrelin receptor by cell based BACMAM FLIPR assay
- ChEMBL_104614 (CHEMBL713592) Functional activity against melatonin receptor in lightening Xenopus laevis tadpole skin
- ChEMBL_104784 (CHEMBL714927) Binding affinity for Melatonin receptor using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104925 (CHEMBL714705) Binding affinity to melatonin receptor measured on ovine pars tuberalis membrane
- ChEMBL_303373 (CHEMBL839691) Binding affinity for human growth hormone secretagogue receptor was determined using [125I]ghrelin
- ChEMBL_306285 (CHEMBL874562) Binding affinity for human growth hormone secretagogue receptor was determined using [125I]ghrelin
- ChEMBL_313051 (CHEMBL835742) Ability to inhibit ghrelin induced increase in intracellular [Ca2+] in CHO-K cells
- ChEMBL_790253 (CHEMBL1925646) Displacement of [125I-His9]-ghrelin from human GRLN receptor by radioligand binding assay
- ChEMBL_104772 (CHEMBL714917) Agonist activity against melatonin receptor was tested in the absence of iodomelatonin
- ChEMBL_104773 (CHEMBL714918) Agonist activity against melatonin receptor was tested in the absence of iodomelatonin
- ChEMBL_2272288 Agonist activity at human melatonin MT1 receptor stably expressing in human HEK293 cells
- ChEMBL_2272289 Agonist activity at human melatonin MT2 receptor stably expressing in human HEK293 cells
- ChEMBL_744413 (CHEMBL1772367) Binding affinity to low affinity melatonin (MT3) site of quinone reductase 2
- ChEMBL_2475653 Displacement of [125I]ghrelin from human GHSR assessed as inhibition constant by competitive binding assay
- ChEMBL_312559 (CHEMBL834350) Inhibition of ghrelin-induced increase of intracellular [Ca2+] in CHO K cell FLIPR assay
- ChEMBL_429455 (CHEMBL917154) Displacement of [125I-His9]ghrelin from human GHS1a receptor expressed in LLC PK1 cells
- ChEMBL_461490 (CHEMBL927501) Displacement of [125I]His9-ghrelin from human GHSR1a receptor expressed in LLC PK1 cells
- ChEMBL_806242 (CHEMBL1958783) Displacement of [125I]-Ghrelin from human GHSR membranes overexpressing GSH-R1a by scintillation counting
- ChEMBL_104764 (CHEMBL714909) Binding affinity against chicken brain melatonin receptors using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104924 (CHEMBL714704) Monophasic inhibitory concentration against melatonin receptor was measured on ovine pars tuberalis membrane.
- ChEMBL_304028 (CHEMBL840206) Binding affinity for human recombinant Melatonin receptor type 2 expressed in NIH3T3 cells
- ChEMBL_304031 (CHEMBL840209) Binding affinity for human recombinant Melatonin receptor type 1 expressed in NIH3T3 cells
- ChEMBL_744409 (CHEMBL1772363) Binding affinity to human low affinity melatonin (MT3) site of quinone reductase 2
- ChEMBL_1499284 (CHEMBL3583166) Antagonist activity at GHSR1a (unknown origin) assessed as inhibition of ghrelin-induced intracellular calcium mobilization
- ChEMBL_1505779 (CHEMBL3595017) Inverse agonist activity rat ghrelin receptor expressed in HEK293 cells by luciferase reporter gene assay
- ChEMBL_509093 (CHEMBL1007237) Displacement of [35S]MK677 from human ghrelin receptor expressed in african green monkey COS7 cells
- ChEMBL_563166 (CHEMBL964270) Displacement of [125I]ghrelin from human growth hormone secretagogue receptor expressed in CHO-K1 cells
- ChEMBL_72506 (CHEMBL685171) Binding affinity towards human pituitary Growth hormone secretagogue receptor using [125I][Tyr4]-ghrelin as radioligand
- ChEBML_104948 Binding affinity against human Melatonin receptor type 1A by using 2-[125I]iodomelatonin as radioligand
- ChEBML_105103 Displacement of 2-[125I]iodomelatonin from human Melatonin receptor type 1A expressed in CHO cells
- ChEBML_105267 Compound was tested for binding affinity against human Melatonin receptor type 1B in CHO cells
- ChEBML_105276 Binding affinity against human Melatonin receptor type 1B by using 2-[125I]iodomelatonin as radioligand
- ChEBML_219514 Binding affinity measured against Chicken brain melatonin receptor by using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104759 (CHEMBL710991) Effect of compound on Melatonin receptor in chicken brain in the presence of MnCl2
- ChEMBL_514503 (CHEMBL973545) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor expressed in CHO cells
- ChEMBL_514504 (CHEMBL973546) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor expressed in CHO cells
- ChEMBL_544427 (CHEMBL1016924) Displacement of [125I]iodomelatonin from MT3/QR2 melatonin binding site expressed in CHO cells
- ChEMBL_1868770 (CHEMBL4369836) Antagonist activity at SNAP-tagged human GHSR expressed in HEK293T cells assessed as reduction in ghrelin-induced inositol phosphate production at 1 uM by measuring ghrelin EC50 after 30 mins by HTRF assay (Rvb = 0.36 +/- 0.01 nM)
- ChEMBL_1868771 (CHEMBL4369837) Antagonist activity at SNAP-tagged human GHSR expressed in HEK293T cells assessed as reduction in ghrelin-induced inositol phosphate production at 1 uM by measuring ghrelin EC50 after 30 mins by HTRF assay (Rvb = 0.40 +/- 0.05 nM)
- ChEMBL_1500295 (CHEMBL3588719) Inverse agonist activity at rat ghrelin receptor expressed in HEK293 cells by inositol phosphate turnover assay
- ChEMBL_104753 (CHEMBL712436) Binding affinity towards melatonin receptor using 2-[125I]iodomelatonin as radioligand in chick brain membranes
- ChEMBL_104779 (CHEMBL714924) Inhibitory activity against melatonin receptor of quail optica tecta with 200 pM 2-[125] iodomelatonin
- ChEMBL_104785 (CHEMBL714928) Inhibition of 2-[125I]iodomelatonin binding to melatonin receptor in quail brain as 1/Ka
- ChEMBL_105267 (CHEMBL713139) Compound was tested for binding affinity against human Melatonin receptor type 1B in CHO cells
- ChEMBL_219514 (CHEMBL824491) Binding affinity measured against Chicken brain melatonin receptor by using 2-[125I]iodomelatonin as radioligand
- ChEMBL_1499282 (CHEMBL3583164) Displacement of 125I-[His9]-ghrelin from human GHSR1a expressed in LLC-PK1 cells by competitive binding assay
- ChEMBL_827488 (CHEMBL2051345) Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA
- ChEMBL_862706 (CHEMBL2174254) Displacement of [35S]MK677 from human ghrelin receptor expressed in COS-7 cells incubated for 3 hrs
- ChEMBL_1868772 (CHEMBL4369838) Antagonist activity at human SNAP-tagged GHSR expressed in HEK293T cells assessed as reduction in ghrelin-induced calcium production at 1 uM by measuring ghrelin EC50 after 15 mins by Fluo 4-AM dye-based fluorescence assay (Rvb = 0.41 +/- 0.13 nM)
- ChEMBL_1868773 (CHEMBL4369839) Antagonist activity at human SNAP-tagged GHSR expressed in HEK293T cells assessed as reduction in ghrelin-induced calcium production at 1 uM by measuring ghrelin EC50 after 15 mins by Fluo 4-AM dye-based fluorescence assay (Rvb = 0.34 +/- 0.12 nM)
- ChEMBL_104609 (CHEMBL712996) Effective concentration against human MT2 (Melatonin) receptor stably expressed in NIH3T3 cells in adenylyl cyclase assay
- ChEMBL_104758 (CHEMBL710990) Effect of compound on Melatonin receptor in chicken brain in the presence of GTP-gamma-S
- ChEMBL_104942 (CHEMBL713109) Inhibition of 2-[125I]iodomelatonin binding to Melatonin receptor 3 (MT3) of Syrian hamster brain membrane
- ChEMBL_104950 (CHEMBL713259) Inhibition of 2-[125I]iodomelatonin binding to human Melatonin receptor type 1A expressed in HEK293 cells
- ChEMBL_105104 (CHEMBL715948) Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1A expressed in CHO cells
- ChEMBL_105269 (CHEMBL713141) Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1B expressed in CHO cells
- ChEMBL_105404 (CHEMBL710823) Inhibition of 2-[125I]iodomelatonin binding to human Melatonin receptor type 1B expressed in HEK293 cells
- ChEMBL_303517 (CHEMBL839631) Binding affinity against Melatonin receptor type 1A stably expressed in NIH3T3 cells using 2-[125I]iodomelatonin
- ChEMBL_303518 (CHEMBL839632) Binding affinity against Melatonin receptor type 1B stably expressed in NIH3T3 cells using 2-[125I]iodomelatonin
- ChEMBL_512176 (CHEMBL968035) Agonist activity at CPR119 transfected in Xenopus dermal melanophore assessed as dispersion of melatonin-induced pigmentation
- ChEBML_89487 In vitro binding affinity against human type 1a growth hormone secretagogue receptor (hGHS-R1a), using [125I]ghrelin as radioligand.
- ChEMBL_1461883 (CHEMBL3396113) Displacement of [125I]Ghrelin from human GHS-R1a expressed in HEK293 cells after 8 hrs by SPA method
- ChEMBL_1500294 (CHEMBL3588718) Inverse agonist activity at rat ghrelin receptor expressed in HEK293 cells by NFAT-RE-luciferase reporter gene assay
- ChEMBL_312888 (CHEMBL826436) Ability to inhibit ghrelin induced increase in intracellular [Ca2+] in CHO-K cells was determined by FLIPR assay
- ChEMBL_858883 (CHEMBL2167929) Displacement of [125I]ghrelin expressed in in LLC-PK1 cells incubated for 1 hr by gamma counting method
- ChEMBL_860219 (CHEMBL2169160) Displacement of [125I]-ghrelin from human GHSR1 expressed in CHO-CREluc cells after 1 hr by scintillation counting
- ChEBML_105098 Binding affinity against human Melatonin receptor type 1A by displacement of [125I]iodomelatonin stably expressed in CHO cells
- ChEBML_105100 Binding of 2-[125I]iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A
- ChEBML_105262 Binding affinity against human Melatonin receptor type 1B by displacement of [125I]iodomelatonin stably expressed in CHO cells
- ChEBML_105264 Binding of 2-[125I]iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B
- ChEMBL_104611 (CHEMBL713590) Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay.
- ChEMBL_104754 (CHEMBL712437) Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay
- ChEMBL_104756 (CHEMBL712439) Binding affinity towards melatonin receptor was determined using 2-[125I]iodomelatonin as radioligand in chick brain membranes
- ChEMBL_104757 (CHEMBL712440) Competitive binding by the displacement of 2-[125I]- Iodomelatonin binding from melatonin receptors in chicken brain membranes
- ChEMBL_104760 (CHEMBL710992) In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes
- ChEMBL_104776 (CHEMBL714921) Inhibitory activity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as labelled ligand
- ChEMBL_104777 (CHEMBL714922) Inhibitory activity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as radiolabeled ligand
- ChEMBL_104783 (CHEMBL714926) Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as labelled ligand
- ChEMBL_104940 (CHEMBL714719) Ability to inhibit 2-[125I]iodomelatonin specific binding to melatonin receptor 3 (MT3) of Syrian hamster brain.
- ChEMBL_105108 (CHEMBL856174) Melatonin receptor type 1A binding affinity measured using 2-[125I]iodomelatonin on ovine pars tuberalis membrane homogenates.
- ChEMBL_1286910 (CHEMBL3110824) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor expressed in CHO cells after 120 mins
- ChEMBL_1286911 (CHEMBL3110825) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor expressed in CHO cells after 120 mins
- ChEMBL_1560583 (CHEMBL3777315) Displacement of 2-[125I]Iodomelatonin from human MT1 melatonin receptor expressed in HEK cells after 120 mins
- ChEMBL_1560584 (CHEMBL3777316) Displacement of 2-[125I]Iodomelatonin from human MT2 melatonin receptor expressed in HEK cells after 120 mins
- ChEMBL_304060 (CHEMBL839735) Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor MT2 expressed in NIH3T3 rat fibroblast cells
- ChEMBL_430239 (CHEMBL914478) Antagonist activity at GHSR expressed in CHOK cells assessed as inhibition of ghrelin-induced increase in intracellular calcium level
- ChEMBL_446527 (CHEMBL895698) Displacement of [125I]ghrelin from ovine recombinant GHSR1a expressed in HEK293F cells after 6 hrs by scintillation proximity assay
- ChEMBL_461492 (CHEMBL927503) Antagonist activity at human GHSR1a receptor expressed in CHO cells assessed as reduction of ghrelin-induced intracellular calcium mobilization
- ChEMBL_519559 (CHEMBL942682) Activity at ghrelin receptor in New Zealand White rabbit gastric antral smooth muscle by calcium mobilization-based FLIPR assay
- ChEMBL_619834 (CHEMBL1106711) Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-stimulated intracellular calcium mobilization
- ChEMBL_105109 (CHEMBL715952) Binding affinity for melatonin 1A receptor was measured using 2-[125I]iodomelatonin on ovine pars tuberalis membrane homogenates.
- ChEMBL_105244 (CHEMBL712574) Binding affinity for melatonin receptor type 1B, expressed in HEK293 cells (2-[125I]iodomelatonin is used as radioligand)
- ChEMBL_105273 (CHEMBL713144) Binding affinity towards melatonin receptor type 1B stably expressed in NIH3T3 rat fibroblast cells using 2-[125I]iodomelatonin
- ChEMBL_1499384 (CHEMBL3583713) Displacement of 2-[125I]-iodomelatonin from human melatonin receptor-1 transfected in CHO cell membranes after 120 mins
- ChEMBL_1499385 (CHEMBL3583714) Displacement of 2-[125I]-iodomelatonin from human melatonin receptor-2 transfected in CHO cell membranes after 120 mins
- ChEMBL_1868767 (CHEMBL4369833) Displacement of [125I]-His9 ghrelin from SNAP-tagged human GHSR expressed in HEK293T cells after 3 hrs by HTRF assay
- ChEMBL_72517 (CHEMBL685782) Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) expressed in COS-7 cells
- ChEMBL_862703 (CHEMBL2174251) Activation of human ghrelin receptor expressed in COS 7 cells using [2-3H]myo-inositol by inositol triphosphate turnover assay
- ChEMBL_104613 (CHEMBL884504) The functional activity on melatonin receptor was evaluated by its potency to lighten the skin of Xenopus laevis tadpoles
- ChEMBL_104782 (CHEMBL714925) Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin (100 pM) as labelled ligand
- ChEMBL_104944 (CHEMBL713111) Binding affinity for human melatonin receptor type 1A, expressed in HEK293 cells (2-[125I]iodomelatonin is used as radioligand)
- ChEMBL_104946 (CHEMBL713113) Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.
- ChEMBL_105080 (CHEMBL711325) Agonist potency determined by [35S]GTP gamma-S binding assay using CHO cell lines for Melatonin receptor type 1A
- ChEMBL_105248 (CHEMBL709923) Agonist potency determined by [35S]GTP gamma-S binding assay using CHO cell lines for Melatonin receptor type 1B
- ChEMBL_1499387 (CHEMBL3583716) Agonist activity at human melatonin receptor-1 transfected in CHO cell membranes after 1 hr by GTPgammaS binding assay
- ChEMBL_1499389 (CHEMBL3583718) Agonist activity at human melatonin receptor-2 transfected in CHO cell membranes after 1 hr by GTPgammaS binding assay
- ChEMBL_303582 (CHEMBL828981) Inhibition of 2-[125I]iodomelatonin binding to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A
- ChEMBL_303583 (CHEMBL828124) Inhibition of 2-[125I]iodomelatonin binding to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B
- ChEMBL_304083 (CHEMBL838521) Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor type 1A (MT1) expressed in NIH3T3 rat fibroblast cells
- ChEMBL_304084 (CHEMBL838522) Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor type 1B (MT2) expressed in NIH3T3 rat fibroblast cells
- ChEMBL_310710 (CHEMBL838055) Agonist activity towards human Melatonin receptor type 1A was determined by its ability to inhibit forskolin stimulated cAMP accumulation
- ChEMBL_310711 (CHEMBL838056) Agonist activity towards human Melatonin receptor type 1B was determined by its ability to inhibit forskolin stimulated cAMP accumulation
- ChEMBL_939502 (CHEMBL2328137) Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method
- ChEMBL_939503 (CHEMBL2328138) Displacement of [3H]melatonin from human MT1 receptor expressed in CHO cells after 60 mins by microbeta scintillation method
- ChEMBL_1449409 (CHEMBL3373238) Displacement of [125I]human ghrelin from human GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method
- ChEMBL_1450204 (CHEMBL3375767) Displacement of [125I]human ghrelin from rat GHS-R1a expressed in HEK cell membranes after 60 mins by gamma counting method
- ChEMBL_509095 (CHEMBL1007239) Inverse agonist activity at human ghrelin receptor expressed in african green monkey COS7 cells assessed as constitutively stimulated inositol phosphate accumulation
- ChEMBL_72518 (CHEMBL685783) Ability to displace [125I]ghrelin from cloned human Growth hormone secretagogue receptor type I (GSH1a) receptor expressed in COS-7 cells
- ChEMBL_862908 (CHEMBL2173092) Agonist activity at human ghrelin receptor expressed in COS 7 cells using [2-3H]myo-inositol by inositol triphosphate turnover assay
- ChEMBL_982014 (CHEMBL2428369) Displacement of [125I]-ghrelin from human GHS-R1a expressed in tetracycline inducible HEK293 cells after 8 hrs by liquid scintillation counting
- HS-R1a radioreceptor assay GHS-R1a radioreceptor assay using LLCPK-1 membranes overexpressing GSH-R1a and radioiodinated (1-28) rat ghrelin as tracer.
- Receptor Binding Studies and GHS-R Ca2+ Flux Assay Specific binding was determined by incubation of membranes from GHS-R1a transfected CHO-K cells with 125I-His9-ghrelin in the presence of increasing concentrations of compounds to obtain IC50. Fluorescence emissions from 96 wells were measured simultaneously at excitation and emission wavelength of 488 and 520 nm, respectively. During this time, agonist responses in the absence of ghrelin, or the antagonist effects of compounds on ghrelin-stimulated calcium flux were recorded. Sigmoidal curves were fitted and EC50/IC50 values were determined by GraphPad Prism software. Ghrelin shows an EC50 of 0.2 nM in this assay.
- ChEMBL_105083 (CHEMBL710854) Intrinsic activity of Melatonin receptor type 1A evaluated on [35S]GTP-gamma-S, binding in Chinese hamster ovarian (CHO) cells
- ChEMBL_1722250 (CHEMBL4137250) Displacement of human [125I]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes after 1 hr by radioligand binding assay
- ChEMBL_1978553 (CHEMBL4611688) Displacement of [125I]-ghrelin from human GHS-R1a stably expressed in HEK cell membrane measured after 60 mins by gamma counter method
- ChEMBL_446528 (CHEMBL895699) Agonist activity at human recombinant GHSR1a expressed in HEK293F cells assessed as stimulation of [35S]GTP-gamma-S binding relative to ghrelin
- ChEMBL_615674 (CHEMBL1106019) Inhibition of rat GHSR expressed in CHOK1 cells assessed as inhibition of ghrelin-induced intracellular calcium flux by aequorin flash luminescence assay
- ChEMBL_72519 (CHEMBL685784) Binding affinity towards cloned human Growth hormone secretagogue receptor type I in LLC PK-1 cells using [125I][His9]-ghrelin as radioligand
- ChEMBL_827492 (CHEMBL2051349) Antagonist activity at human GHS-R1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced europium-labeled GTP binding by DELFIA
- ChEMBL_862705 (CHEMBL2174253) Inverse agonist activity at human ghrelin receptor expressed in COS 7 cells using [2-3H]myo-inositol by inositol triphosphate turnover assay
- ChEMBL_104778 (CHEMBL714923) Inhibitory activity against melatonin receptor of quail optica tecta with 200 pM 2-[125] iodomelatonin as gamma-S (10e-4 M)
- ChEMBL_105082 (CHEMBL710853) Intrinsic activity evaluated on [35S]GTP -gamma-S binding in hamster ovarian (CHO) cells, stably expressing human Melatonin receptor type 1A
- ChEMBL_105101 (CHEMBL715945) Binding affinity towards recombinant human melatonin receptor type 1A expressed in NIH 3T3 cells using 2-[121I]iodomelatonin radioligand binding assay
- ChEMBL_105249 (CHEMBL709924) Intrinsic activity at human Melatonin receptor type 1B evaluated on [35S]GTP-gamma-S, binding in Chinese hamster ovarian (CHO) cells
- ChEMBL_105250 (CHEMBL710586) Intrinsic activity at human Melatonin receptor type 1B evaluated on [35S]GTP-gamma-S, binding in Chinese hamster ovarian (CHO) cells
- ChEMBL_105265 (CHEMBL872558) Binding affinity towards recombinant human melatonin receptor type 1B expressed in NIH 3T3 cells using 2-[121I]iodomelatonin radioligand binding assay
- ChEMBL_1499416 (CHEMBL3583851) Agonist activity at human recombinant melatonin receptor-1 expressed in CHO cells assessed as effect on impedance by cellular dielectric spectroscopy
- ChEMBL_1992276 (CHEMBL4626011) Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method
- ChEMBL_2349023 Displacement of his-tagged [125I]-ghrelin from eYFP-labelled GHSR1a (unknown origin) transfected in HEK293 cells incubated for 20 mins by radioligand binding assay
- ChEMBL_615667 (CHEMBL1106012) Antagonist activity at human GHSR expressed in CHOK1 cells assessed as inhibition of ghrelin-induced intracellular calcium flux by aequorin flash luminescence assay
- ChEMBL_862700 (CHEMBL2174248) Agonist activity at FLAG-tagged human ghrelin receptor expressed in COS 7 cells using [2-3H]myo-inositol by inositol triphosphate turnover assay
- ChEMBL_105081 (CHEMBL711326) Intrinsic activity evaluated on [35S]GTP -gamma-S binding in Chinese hamster ovarian (CHO) cells, stably expressing human Melatonin receptor type 1A
- ChEMBL_105099 (CHEMBL714106) Binding affinity on human melatonin receptor type 1A stably transfected in human embryonic kidney (HEK 293) using 2-[125I]iodomelatonin as radioligand.
- ChEMBL_105112 (CHEMBL715955) Binding affinity towards melatonin receptor type 1A stably expressed in NIH3T3 rat fibroblast cells using 2-[125I]iodomelatonin (100 pM) as radioligand
- ChEMBL_1574205 (CHEMBL3802020) Displacement of [125I]-His9-ghrelin from human GHS-R1a transfected in pig LLC PK-1 cell membranes after 60 mins by gamma counting method
- ChEMBL_2063396 (CHEMBL4718649) Displacement of [125I-His9]-ghrelin from GHS-R1a (unknown origin) expressed in human HEK293 cells incubated for 20 mins by gamma scintillation counting method
- ChEMBL_509373 (CHEMBL1001327) Agonist activity at human wild type ghrelin receptor expressed in african green monkey COS7 cells assessed as stimulation of constitutively stimulated inositol phosphate accumulation
- ChEMBL_862726 (CHEMBL2174351) Inverse agonist activity at FLAG-tagged human ghrelin receptor expressed in COS 7 cells using [2-3H]myo-inositol by inositol triphosphate turnover assay
- ChEMBL_105097 (CHEMBL714104) Binding affinity for human Melatonin receptor type 1A stably transfected in human embryonic kidney cells (HEK 293) using 2-[125I]iodomelatonin as radioligand
- ChEMBL_105261 (CHEMBL710751) Binding affinity for human Melatonin receptor type 1B stably transfected in human embryonic kidney cells (HEK 293) using 2-[125I]iodomelatonin as radioligand
- ChEMBL_105263 (CHEMBL715855) Binding affinity on human melatonin receptor type 1B stably transfected in human embryonic kidney (HEK 293) cells using 2-[125I]iodomelatonin as radioligand.
- ChEMBL_2026951 (CHEMBL4681109) Agonist activity at MT1 (unknown origin) expressed in HEK293T cells incubated for 15 mins by melatonin Gi/o-mediated cAMP inhibition GloSensor assay
- ChEMBL_2026952 (CHEMBL4681110) Agonist activity at MT2(unknown origin) expressed in HEK293T cells incubated for 15 mins by melatonin Gi/o-mediated cAMP inhibition GloSensor assay
- Pharmacological Activity Each assay plate contains wells with vehicle controls (1% DMSO) for the measurement of non-inhibited transfer reaction (=100% Ctl) and wells with 10 μM ([Dap3]-Ghrelin) as controls for fully inhibited GOAT enzymeThe analysis of the data is performed by calculation of the percentage of acyl-ghrelin produced in the presence of test compound compared to the amount of acyl-ghrelin produced in the vehicle control samples. An inhibitor of the GOAT enzyme will give values between 100% CTL (no inhibition) and 0% CTL (complete inhibition).
- ChEMBL_2207826 (CHEMBL5120534) Binding affinity to Ghrelin in human serum expressed in HEK293T cells using NanoLuc as substrate measured after 1 hr by bioluminescence based microplate reader assay
- ChEMBL_509091 (CHEMBL1007235) Inverse agonist activity at human wild type ghrelin receptor expressed in african green monkey COS7 cells assessed as inhibition of constitutively stimulated inositol phosphate accumulation
- ChEMBL_1499414 (CHEMBL3583849) Agonist activity at human recombinant melatonin receptor-2 expressed in CHO cells assessed as decrease of cAMP level after 10 mins by HTRF assay
- ChEMBL_1574207 (CHEMBL3802212) Antagonist activity at human GHS-R1a transfected in mouse LTK cells after 6 hrs by CRE/luciferase reporter gene assay in presence of ghrelin and rolipram
- ChEMBL_2207823 (CHEMBL5120531) Displacement of C-terminal Cys-tagged SmBiT tracer from C-terminal Cys-tagged human Ghrelin expressed in HEK293T cells by microplate reader based NanoBiT-binding assay
- ChEMBL_2207825 (CHEMBL5120533) Displacement of C-terminal Cys-tagged NanoLuc luciferase from C-terminal Cys-tagged human Ghrelin expressed in HEK293T cells by microplate reader based NanoBiT-binding assay
- ChEMBL_2207828 (CHEMBL5120536) Binding affinity to Ghrelin in fetal bovine serum expressed in HEK293T cells using NanoLuc as substrate measured after 1 hr by bioluminescence based microplate reader assay
- ChEMBL_2016801 (CHEMBL4670379) Inverse agonist activity at SNAP-tagged human GHSR expressed in HEK293 cells assessed as inhibition of ghrelin-induced IP1 response measured after 30 mins by HTRF assay
- ChEMBL_2228632 (CHEMBL5142145) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor stably expressing in human HEK293 cell membranes under dark condition by radioligand based competition binding assay
- ChEMBL_2228635 (CHEMBL5142148) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor stably expressing in human HEK293 cell membranes under dark condition by radioligand based competition binding assay
- ChEMBL_1499283 (CHEMBL3583165) Antagonist activity at human GSHR1a expressed in mouse LTK cells assessed as inhibition of ghrelin-induced reporter gene expression after 6 hrs by CRE/luciferase reporter gene assay
- ChEMBL_2019849 (CHEMBL4673662) Inhibition of human His-tagged GOAT expressed in baculovirus infected Sf9 cell membrane using desacyl-ghrelin-biotin and octanoyl-CoA as substrate incubated for 1 hr by ELISA
- ChEMBL_2207824 (CHEMBL5120532) Displacement of C-terminal Cys-tagged human LEAP2-SmBiT tracer from C-terminal Cys-tagged human Ghrelin expressed in HEK293T cells by microplate reader based NanoBiT-binding assay
- ChEMBL_2019855 (CHEMBL4673668) Inhibition of human GOAT expressed in baculovirus infected sf9 insect cell microsomes using biotinylated des-acyl ghrelin and octanoyl-CoA as substrate incubated for 15 mins by HTRF assay
- Binding to Melatonin MT3 Binding Sites The experiment of binding to MT3 sites was carried out on hamster brain membranes using [125I]2-iodomelatonin as radioligand in accordance with the protocol described by Pickering, D. S et al. (Pickering, D. S et al, 1990, Pharmacological characterization of melatonin binding sites in Syrian hamster hypothalamus, Eur J Pharmacol. 1990 Jan. 3; 175(1):71-7).
- ChEMBL_1574206 (CHEMBL3802211) Antagonist activity at human GHS-R1a transfected in CHO cells assessed as inhibition of ghrelin-induced intracellular calcium mobilization measured over 60 secs by fluo-4AM dye-based fluorometric method
- ChEMBL_2016806 (CHEMBL4670384) Displacement of red-ghrelin from SNAP-tagged human GHSR expressed in HEK293 cells incubated for 3 hrs at 4 degC or for 1 hr at room temperature by HTRF assay
- ChEMBL_2019854 (CHEMBL4673667) Inhibition of mouse GOAT expressed in baculovirus infected sf9 insect cell microsomes using biotinylated des-acyl ghrelin and octanoyl-CoA -CoA as substrate incubated for 15 mins by HTRF assay
- Receptor Binding Assay with 125I-Ghrelin Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increasing concentrations of unlabeled ghrelin or with increasing concentrations of azapeptides derivatives in 0.5 mL of binding buffer. Bound radiolabelled ligand was separated from free by filtration on Whatman GF/C filters pretreated with a 1% polyethylenimine solution. Filters were washed with 2×3 mL of washing buffer (50 mM Tris-HCl, pH 7.3, 10 mM MgCl2, 2.5 mM EDTA, 0.015% Triton X-100). Filters were counted in a LKB gamma counter. Curves were analyzed using Prism software (GraphPad Software Inc).
- ChEMBL_2228633 (CHEMBL5142146) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor stably expressing in human HEK293 cell membranes in presence of light-activated compounds by radioligand based competition binding assay
- ChEMBL_2228636 (CHEMBL5142149) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor stably expressing in human HEK293 cell membranes in presence of light-activated compounds by radioligand based competition binding assay
- ChEMBL_1788623 (CHEMBL4260357) Antagonist activity at recombinant human GHSR1a expressed in HEK293 cells assessed as inhibition of ghrelin-induced intracellular calcium release incubated for 20 secs and measured after 2 mins by FLIPR assay
- ChEMBL_2019860 (CHEMBL4673673) Inhibition of human His-tagged GOAT expressed in baculovirus infected Sf9 cell membrane using biotinylated ghrelin peptide/octanoyl-CoA/palmitoyl CoA as substrate incubated for 80 mins by TR-FRET assay
- ChEMBL_2228637 (CHEMBL5142150) Agonist activity at human melatonin MT1 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins under dark condition by cAMP assay
- ChEMBL_2228641 (CHEMBL5142154) Agonist activity at human melatonin MT2 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins under dark condition by cAMP assay
- ChEMBL_2228638 (CHEMBL5142151) Agonist activity at human melatonin MT1 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins in presence of light-activated compounds by cAMP assay
- ChEMBL_2228642 (CHEMBL5142155) Agonist activity at human melatonin MT2 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins in presence of light-activated compounds by cAMP assay
- Receptor Binding Studies Specific binding was determined by incubation of membranes from GHS-R1a transfected LLC cells with 125I-His9-ghrelin in the presence of increasing concentrations of compounds to obtain IC50. For the compounds behaving as agonists and displaying a high affinity binding for hGHS-R1a in radiolabeled binding experiments, the EC50 (the molar concentration of the agonist producing 50% of the maximal possible effect of that agonist) was determined using a dose-response curve. In the case of high-affinity antagonists, Kb values were determined using antagonist inhibition curves in the presence of 0.1 uM ghrelin (submaximal concentration).
- ChEMBL_2019856 (CHEMBL4673669) Antagonist activity at mouse GOAT expressed in baculovirus infected sf9 insect cell membrane using ghrelin(1 to 5) pentapeptide as substrate preincubated for 5 mins followed by Palmitoyl-CoA and n-octynoyl-CoA addition and measured after 5 mins by amplex red/H2O2 reagent-based fluorescence method
- hGOAT Activity in HEK293 Cells after Incubation Cells are plated with a density of 5000 cells/well in 384-well poly-D-lysin plates and incubated for 1 day at 37° C., 5% CO2 in DMEM medium, 10% FCS, 1×NEAA, Puromycin (0.5 μg/ml) and G418 (1 mg/ml). Then the medium is changed to a identical medium without FCS and containing Octanoate-BSA (final concentration 100 μM each) and compound in DMSO (final DMSO concentration 0.3%). After incubation for 5 hours acylghrelin in the medium is measured by ELISAThe medium sample is diluted 1:25 in Elisa buffer, A 25 ul aliquot is transferred to a 384-well ELISA plate previously washed 4 times with 100 μL wash buffer, and 25 μl tracer-solution is added. After incubation overnight ( 20 h) at 4° C. temperature the plate is washed 4 times with 100 μl wash-buffer per well. Finally 50 μl Ellman's reagent is added to each well and the plate is incubated in the dark for 20 minutes. The absorbance is measured at 405 nm in an Envision multilabel reader and the amount of acylated ghrelin is calculated according to a acylated ghrelin standard curve provided in the same plate.Each assay plate contains wells with vehicle controls (1% DMSO) for the measurement of non-inhibited transfer reaction (=100% Ctl) and wells with 10 μM ([Dap3]-Ghrelin) as controls for fully inhibited GOAT enzymeThe analysis of the data is performed by calculation of the percentage of acyl-ghrelin produced in the presence of test compound compared to the amount of acyl-ghrelin produced in the vehicle control samples. An inhibitor of the GOAT enzyme will give values between 100% CTL (no inhibition) and 0% CTL (complete inhibition). IC50 values are calculated with Assay Explorer or other suited software based on curve fitting of results of 8 different compound concentrations.
- Pharmacological Activity Assay Methods: Cells are plated with a density of 5000 cells/well in 384-well poly-D-lysin plates and incubated for 1 day at 37° C., 5% CO2 in DMEM medium, 10% FCS, 1×NEAA, Puromycin (0.5 μg/ml) and G418 (1 mg/ml). Then the medium is changed to a identical medium without FCS and containing Octanoate-BSA (final concentration 100 μM each) and compound in DMSO (final DMSO concentration 0.3%). After incubation for 5 hours acylghrelin in the medium is measured by ELISA. The medium sample is diluted 1:25 in Elisa buffer, a 25 μl aliquot is transferred to a 384-well ELISA plate previously washed 4 times with 100 μL wash buffer, and 25 μl tracer-solution is added. After incubation overnight ( 20 h) at 4° C. temperature the plate is washed 4 times with 100 μl wash-buffer per well. Finally 50 μl Ellman's reagent is added to each well and the plate is incubated in the dark for 20 minutes. The absorbance is measured at 405 nm in an Envision multilabel reader and the amount of acylated ghrelin is calculated according to a acylated ghrelin standard curve provided in the same plate. Each assay plate contains wells with vehicle controls (1% DMSO) for the measurement of non-inhibited transfer reaction (=100% Ctl) and wells with 10 μM ([Dap3]-Ghrelin) as controls for fully inhibited GOAT enzyme.
- Fluorescence Imaging Plate Reader (FLIPR) Assay The functional FLIPR assay was developed from H4 glioma cells in which expression of the endogenous human GHS receptor was enhanced by RAGE-activation. The assay utilized the GHS receptor natural signaling through a calcium phospholipase C mediated pathway; agonists could be tested for their ability to stimulate intracellular calcium mobilization using a calcium-sensitive fluorescent probe. The EC50 was measured by determining the intracellular calcium concentration in a FLIPR assay, with ghrelin serving as the full agonist reference standard. All of the compounds were determined to be full agonist of the receptor.
- GHS Ligand Binding Assay Briefly, direct ligand binding experiments were performed using fluorescence energy transfer with the purified receptor labeled with Alexa Fluor 350 at its N terminus and a ghrelin peptide labeled with FITC (JMV 4946). Titration experimentswere carried out with protein concentrations in the 10 nM protein concentration range and increasing ligand concentrations. Competition experiments were carried out by adding increasing concentrations of the competing compound to a receptor-JMV 4946 mixture (100 nM concentration range). Fluorescence emission spectra were recorded at 20 °C between 400 and 600 nm on a Cary Eclipse spectrofluorimeter (Varian) with an excitation at 346 or 488 nm. Buffer contributions were systematically subtracted. The FRET ratio corresponds to the ratio of theacceptor-emitted fluorescence at 520 nm from excitation attwo different wavelengths, 346 and 488 nm.
- GOAT Activity Assay Assays were performed with ~100 μg of membrane protein, as determined by a Bradford assay. The membrane fraction was preincubated with 1 μM methyl arachidonyl fluorophosphonate (MAFP) and inhibitor or vehicle as indicated in 50 mM HEPES (pH 7.0) for 30 min at room temperature [McGovern-Gooch et al., Endocrinology, 157:4330-4338]. Reactions were initiated via the addition of 500 μM octanoyl CoA and 1.5 μM fluorescently labeled ghrelin mimetic, GSSFLCAcrylodan, for a total volume of 50 μL, and were incubated for 3 h at room temperature in the dark. Reactions were stopped with the addition of 50 μL of 20% acetic acid in isopropanol, and solutions were clarified by proteinprecipitation with 16.7 μL of 20% trichloroacetic acid, followed by centrifugation (1000g for 1 min). The supernatant was then analyzed by reverse phase HPLC, as previously described [Darling et al., Anal. Biochem., 437:68-76].
- GOAT activity assay GOAT activity was assessed using a time-resolved fluorescence energy transfer (TR-FRET) assay in a 384-well format. His-tag human GOAT enzyme was in the form of a cell membrane preparation from sf9 cells infected with hGOAT-V5-His baculovirus. Varying concentrations of test compound with final DMSO concentration kept to 0.5% were added to membrane solution. Human GOAT membrane activity was established in a buffer having final concentration 0.25 mg/mL in 50 mM MOPS, pH7.5; 50 mM KCl; 0.1 mg/mL BSA; 50 μM CHAPS; and 2 mM EDTA. Substrate solution consisting of biotinylated ghrelin peptide (final concentration 100 nM), octanoyl coA (final concentration 2 μM) and palmitoyl CoA (final concentration 50 μM) was added to initiate the reaction. Plates were sealed, centrifuged for 1 minute at 2000 rpm, then incubated at 30° C. for 80 minutes with gentle shaking on an Eppendorf mix plate. Reaction termination and detection mix consisting of chicken anti-active ghrelin antibody (final concentration of 10 nM), Europium W1024-labeled streptavidin (final concentration of 4 nM), GOAT anti-chicken Dylight (final concentration of 12.5 nM), and GS[DAP-oc]-FL-amide inhibitor (final concentration of 1 μM) was added before further incubation for 40 minutes at 30° C. The plate was then read on an Envision in HTRF mode with excitation filter UV (TRF) 340 and first emission filter of APC 665 and a second emission filter of Europium 615. HTRF readings were acquired as per instrument defined LANCE-DELFIA protocol with a delay and window times of 50 μs for both; number of sequential windows: 1; time between flashes: 2000 μs between each of 100 flashes and 10 flashes for the second detector. The HTRF ratio was calculated directly by the instrument as the ratio of 665 window/615 window. Percent inhibition was calculated as 100−(100×(U−NC)/(PC−NC)) where U was the unknown value HTRF ratio (test compound value), NC was the negative control (100% inhibition value generated from a potent inhibitor), and PC was the positive control (100% activity generated from 0.5% DMSO vehicle). IC50 values were generated in GraphPad Prism (Version 4.03) using non-linear regression curve fit and sigmoidal dose response variable slope analysis.
- Human GOAT Enzymatic Assay Prepare test compounds in DMSO to make up a 0.2 mM stock solution. Serially dilute the stock solution in DMSO for ten concentrations with final compound concentrations ranging from 10 μM to 0.5 nM in a 96-well round-bottom plate. Prepare enzyme and substrate solutions in assay buffer (0.02% TWEEN™-20 in 50 mM Tris, pH 7.5 containing 250 mM sucrose, 1 mg/mL BSA and 10 mM EDTA). Add diluted compound (1 μL) to each well of row A to N of a corresponding low protein binding 384 well plate. Add human GOAT substrate mix (10 μL), consisting of human desacyl-ghrelin-biotin (CPC Scientific Inc., 6.0 μM final), octanoyl-CoA (Sigma, 60 μM final) and an AG specific antibody (WO 2006/091381) (1.0 μg/mL final), to the compounds. Add GOAT-His/sf9 enzyme preparation, that has been prepared in assay buffer (9 μL), to each well of the plate containing substrate and test compounds resulting in a final concentration of 0.01 μg/mL to initiate the reaction. Incubate the mixture for 1 h at RT on a gently rotating oscillator. Add 4 M guanidine hydrochloride (20 μL) to all wells, mix, and incubate for 3 h to stop the reaction.Prepare ELISA plates (STREPTAVIDIN SPECTRAPLATE 384, Perkin Elmer) by blocking with 2% Heat-Inactivated FBS in PBS (40 μL) (Invitrogen) blocking buffer for 3 h. Aspirate the blocking buffer from ELISA plate and add blocking buffer (23 μL) to columns 1-24, rows A-N. Reserve rows O and P for the acylghrelin standard curve. Add the reaction mixture (2 μL) to the ELISA plates. Prepare a 10 point standard curve (biotin-labeled octanoyl-ghrelin) by serial 2× dilution in blocking buffer containing 0.2M Guanidine hydrochloride starting at 2.5 μM. Incubate the reaction mixture or biotin-labeled AG standard in the ELISA plate overnight at 4° C. The following day, wash the plate 3× with wash buffer (0.1% TWEEN-20/PBS, 100 μL per well in each wash cycle). Add AG specific antibody (WO 2006/091381) (25 μL of 0.5 μg/mL in blocking buffer) to each well and incubate at RT for 1 h. Wash the plate 3× with the wash buffer, similarly to the previous step. Add Protein G-HRP (25 μL) (Southern Biotech) diluted 3,000× in blocking buffer and incubate 1 h at RT. Wash the late 3× with wash buffer, as in the previous steps. Add TMB reagent (25 μL) (Kirkegaard & Perry Laboratories, Inc.) to each well and let develop for 20 min and stop with 1 M phosphoric acid (25 μL per well). Read plates at 450 nm using an ENVISION Multilabel plate reader. AG levels are calculated versus a fitted standard curve and percent inhibition calculated. The 10-point inhibition curve is plotted and fitted with the four-parameter logistic equation to obtain IC50 values using ACTIVITYBASE (ver. 7.3.2.1).Following a protocol essentially as described above, all of the compounds of the Examples herein were tested and exhibited an IC50 for the in vitro cell free human GOAT enzymatic assay of lower than 1 μM.
- Human GOAT Enzymatic Assay Prepare test compounds in DMSO to make up a 0.2 mM stock solution. Serially dilute the stock solution in DMSO to obtain a ten-point dilution curve with final compound concentrations ranging from 10 μM to 0.5 nM in a 96-well round-bottom plate. Prepare enzyme and substrate solutions in assay buffer (0.02% TWEEN-20 in 50 mM Tris, pH 7.5/250 mM sucrose/1 mg/mL BSA/10 mM EDTA). Add diluted compound (1 μL) to each well of row A to N of a corresponding low protein binding 384 well plate. Add human GOAT substrate mix (10 μL), consisting of human desacyl-ghrelin-biotin (CPC Scientific Inc., 6.0 μM final), octanoyl-coenzyme A (CoA) (Sigma, 60 μM final) and an AG specific antibody (WO 2006/091381)(1.0 fig/mL final), to the compounds. Add GOAT-His/sf9 enzyme preparation, that has been prepared in assay buffer (9 μL), to each well of the plate containing substrate and test compounds resulting in a final concentration of 0.01 μg/mL to initiate the reaction. Incubate the mixture for 1 hour at RT on a gently rotating oscillator. Add 4M guanidine hydrochloride (20 μL) to all wells, mix, and incubate for 3 hours to stop the reaction. Prepare ELISA plates (STREPTAVIDIN SPECTRAPLATE 384, Perkin Elmer) by blocking with 2% Heat-Inactivated FBS in PBS (40 μL) (Invitrogen) blocking buffer for 3 hours. Aspirate the blocking buffer from ELISA plate and add blocking buffer (23 μL) to columns 1-24, rows A-N. Reserve rows O and P for the acylghrelin standard curve. Add the reaction mix (2 μL) to the ELISA plates. Prepare a 10 point standard curve (biotin-labeled octanoyl-ghrelin) by serial 2× dilution in blocking buffer containing 0.2M Guanidine hydrochloride starting at 2.5 pM. Incubate the reaction mixture or biotin-labeled AG standard in the ELISA plate overnight at 4° C. The following day, wash the plate 3× with wash buffer (0.1% TWEEN-20/PBS, 100 μL per well in each wash cycle). Add AG specific antibody (WO 2006/091381) (25 μL of 0.5 μg/mL in blocking buffer) to each well and incubate at RT for 1 hour. Wash the plate 3× with the wash buffer, similarly to the previous step. Add Protein G-HRP (25 μL)(Southern Biotech) diluted 3,000× in blocking buffer and incubate 1 hour at RT. Wash the late 3× with wash buffer, as in the previous steps. Add TMB reagent (25 μL) (Kirkegaard & Perry Laboratories, Inc.) to each well and let develop for 20 min and stop with 1M phosphoric acid (25 μL per well). Read plates at 450 nm using an ENVISION Multilabel plate reader. AG levels are calculated versus a fitted standard curve and percent inhibition calculated. The 10-point inhibition curve is plotted and fitted with the four-parameter logistic equation to obtain IC50 values using ACTIVITYBASE (ver. 7.3.2.1). Following a protocol essentially as described above, all of the compounds of the Examples herein were tested and exhibited an IC50 for the in vitro cell free human GOAT enzymatic assay of lower than 1 μM.
 US20240190875, Example Ghrelin BDBM21940 Ghrelin
US20240190875, Example Ghrelin BDBM21940 Ghrelin GHRELIN BDBM50366689
GHRELIN BDBM50366689 BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy
BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy BDBM85063 Melatonin,6-Cl
BDBM85063 Melatonin,6-Cl Melatonin,2-Chloro BDBM85236
Melatonin,2-Chloro BDBM85236 melatonin, 6-Methoxy BDBM82556
melatonin, 6-Methoxy BDBM82556 CHEMBL428282 Des-acyl ghrelin BDBM50163757
CHEMBL428282 Des-acyl ghrelin BDBM50163757 BDBM675521 US20240158438, SEQ ID NO. Ghrelin
BDBM675521 US20240158438, SEQ ID NO. Ghrelin BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo
BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin
CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me
BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622
CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622 BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin
BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4
BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4 BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide
BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy
BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110
Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110 CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin)
CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin) N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730
N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide
BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014
N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014 Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide
Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a
6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a
BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide
BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010
Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide
7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide
BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a
6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide
BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide