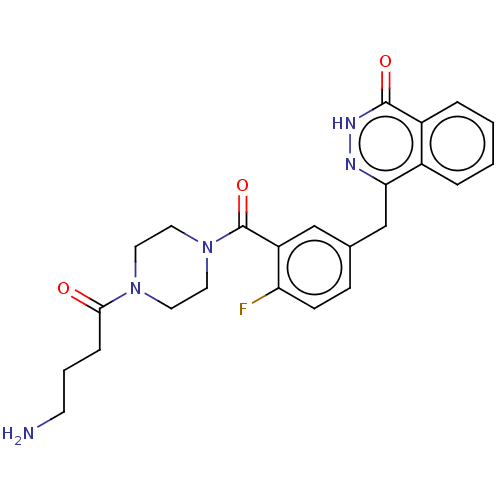
 BDBM209930 c-Olaparib
BDBM209930 c-Olaparib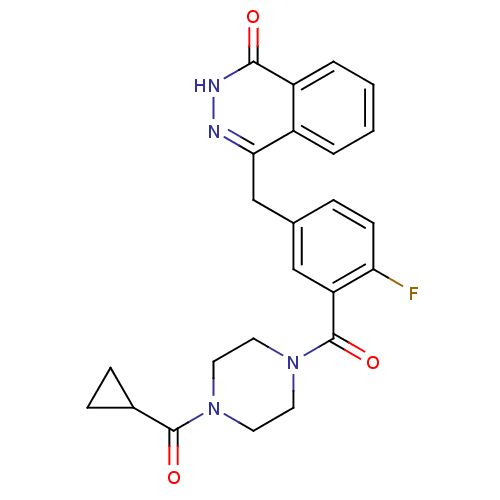 US10174023, AZD2281 US9187430, AZD-2281 Acylpiperazine analogue, 47 4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbonyl]-4-fluorophenyl}methyl)-1,2-dihydrophthalazin-1-one Olaparib US9255106, AZD2281 BDBM27566 US10597399, Compound AZD2281
US10174023, AZD2281 US9187430, AZD-2281 Acylpiperazine analogue, 47 4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbonyl]-4-fluorophenyl}methyl)-1,2-dihydrophthalazin-1-one Olaparib US9255106, AZD2281 BDBM27566 US10597399, Compound AZD2281
- Falchi, F; Giacomini, E; Masini, T; Boutard, N; Di Ianni, L; Manerba, M; Farabegoli, F; Rossini, L; Robertson, J; Minucci, S; Pallavicini, I; Di Stefano, G; Roberti, M; Pellicciari, R; Cavalli, A Synthetic Lethality Triggered by Combining Olaparib with BRCA2-Rad51 Disruptors. ACS Chem Biol 12: 2491-2497 (2017)
- Wong, WW; O'Brien-Gortner, SF; Anderson, RF; Wilson, WR; Hay, MP; Dickson, BD Hypoxia-activated prodrugs of phenolic olaparib analogues for tumour-selective chemosensitisation. RSC Med Chem 14: 1309-1330 (2023)
- Yuan, Z; Chen, S; Sun, Q; Wang, N; Li, D; Miao, S; Gao, C; Chen, Y; Tan, C; Jiang, Y Olaparib hydroxamic acid derivatives as dual PARP and HDAC inhibitors for cancer therapy. Bioorg Med Chem 25: 4100-4109 (2017)
- Roberti, M; Schipani, F; Bagnolini, G; Milano, D; Giacomini, E; Falchi, F; Balboni, A; Manerba, M; Farabegoli, F; De Franco, F; Robertson, J; Minucci, S; Pallavicini, I; Di Stefano, G; Girotto, S; Pellicciari, R; Cavalli, A Rad51/BRCA2 disruptors inhibit homologous recombination and synergize with olaparib in pancreatic cancer cells. Eur J Med Chem 165: 80-92 (2019)
- Reilly, SW; Puentes, LN; Wilson, K; Hsieh, CJ; Weng, CC; Makvandi, M; Mach, RH Examination of Diazaspiro Cores as Piperazine Bioisosteres in the Olaparib Framework Shows Reduced DNA Damage and Cytotoxicity. J Med Chem 61: 5367-5379 (2018)
- Kayumov, M; Jia, L; Pardaev, A; Song, SS; Mirzaakhmedov, S; Ding, C; Cheng, YJ; Zhang, RI; Bao, X; Miao, ZH; He, JX; Zhang, A Design, synthesis and pharmacological evaluation of new PARP1 inhibitors by merging pharmacophores of olaparib and the natural product alantolactone. Eur J Med Chem 240: (2022)
- James, DI; Smith, KM; Jordan, AM; Fairweather, EE; Griffiths, LA; Hamilton, NS; Hitchin, JR; Hutton, CP; Jones, S; Kelly, P; McGonagle, AE; Small, H; Stowell, AI; Tucker, J; Waddell, ID; Waszkowycz, B; Ogilvie, DJ First-in-Class Chemical Probes against Poly(ADP-ribose) Glycohydrolase (PARG) Inhibit DNA Repair with Differential Pharmacology to Olaparib. ACS Chem Biol 11: 3179-3190 (2016)
- Bagnolini, G; Milano, D; Manerba, M; Schipani, F; Ortega, JA; Gioia, D; Falchi, F; Balboni, A; Farabegoli, F; De Franco, F; Robertson, J; Pellicciari, R; Pallavicini, I; Peri, S; Minucci, S; Girotto, S; Di Stefano, G; Roberti, M; Cavalli, A Synthetic Lethality in Pancreatic Cancer: Discovery of a New RAD51-BRCA2 Small Molecule Disruptor That Inhibits Homologous Recombination and Synergizes with Olaparib. J Med Chem 63: 2588-2619 (2020)
- Ren, H; Bakas, NA; Vamos, M; Chaikuad, A; Limpert, AS; Wimer, CD; Brun, SN; Lambert, LJ; Tautz, L; Celeridad, M; Sheffler, DJ; Knapp, S; Shaw, RJ; Cosford, NDP Design, Synthesis, and Characterization of an Orally Active Dual-Specific ULK1/2 Autophagy Inhibitor that Synergizes with the PARP Inhibitor Olaparib for the Treatment of Triple-Negative Breast Cancer. J Med Chem 63: 14609-14625 (2020)
- ChEMBL_2349356 Displacement of Olaparib-BDY FL from PARP1 (unknown origin) incubated for 4 hrs by FP assay
- Biochemical Activity PARP-1: The autoparsylation assay is performed as 384-well HTRF (Cisbio, Codolet, France) assay format in Greiner low volume nb 384-well microtiter plates. 35 nM His-tagged Parp-1 (human, recombinant, Enzo Life Sciences GmbH, L rrach, Germany) and a mixture of 125 nM bio-NAD (Biolog, Life science Inst., Bremen, Germany) and 800 nM NAD as co-substrate are incubated in a total volume of 6 μl (100 mM Tris/HCl, 4 mM Mg-chloride, 0.01% IGEPAL CA630, 1 mM DTT, 0.5% DMSO, pH 8, 13 ng/μl activated DNA (BPS Bioscience, San Diego, US)) in the absence or presence of the test compound (10 dilution concentrations) for 150 min at 23° C. The reaction is stopped by the addition of 4 μl of the Stop/detection solution (70 nM SA-Xlent (Cisbio, Codolet, France), 2.5 nM Anti-His-K (Eu-labelled anti-His, Cisbio, Codolet, France) in 50 mM HEPES, 400 mM KF, 0.1% BSA, 20 mM EDTA, pH 7.0). After 1 h incubation at room temperature the HTRF iss measured with an Envision multimode reader (Perkin Elmer LAS Germany GmbH) at excitation wavelength 340 nm (laser mode) and emission wavelengths 615 nm and 665 nm. The ratio of the emission signals is determined. The full value used is the inhibitor-free reaction. The pharmacological zero value used is Olaparib (LClabs, Woburn, US) in a final concentration of 1 μM.

