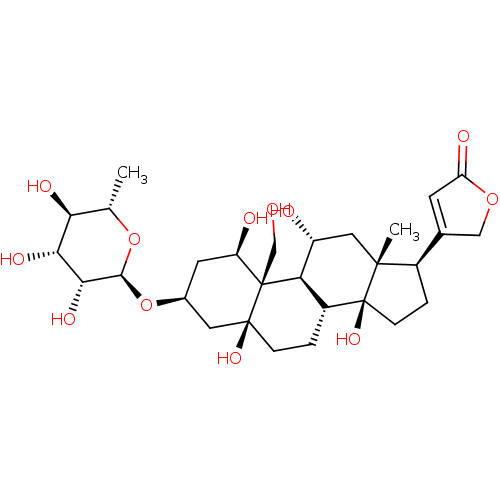
 4-((1R,3S,5S,8R,9S,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[(1R,3S,5S,10R,11R,13R,14S,17R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one Ouabain4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one(Ouabain) Ouabain CHEMBL222863 4-[(R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one cid_439501 BDBM50286739 NSC-25485
4-((1R,3S,5S,8R,9S,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[(1R,3S,5S,10R,11R,13R,14S,17R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one Ouabain4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one(Ouabain) Ouabain CHEMBL222863 4-[(R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one cid_439501 BDBM50286739 NSC-25485
- Xie, Z; Shapiro, JI; Si, S; Zhang, Z Na/K-ATPase ligands, ouabain antagonists, assays and uses thereof US Patent US9114126 (2015)
- Syeda, SS; Sánchez, G; Hong, KH; Hawkinson, JE; Georg, GI; Blanco, G Design, Synthesis, and in Vitro and in Vivo Evaluation of Ouabain Analogues as Potent and Selective Na,K-ATPaseα4 Isoform Inhibitors for Male Contraception. J Med Chem 61: 1800-1820 (2018)
- ChEMBL_54059 (CHEMBL669632) Inhibition of [3H]ouabain binding to Digitalis receptor in dog heart microsomes
- ChEMBL_144101 (CHEMBL751505) Compound was evaluated for its ability to inhibit the specific [3H]-ouabain binding to dog kidney Na+/K+ ATPase
- ChEMBL_1571199 (CHEMBL3795960) Inhibition of rat ROMK assessed as thallium flux after 30 mins in presence of ouabain by cell based FLIPR assay
- Na,K-ATPase Activity Assay Na,K-ATPase activity was assayed in vitro by measuring the release of 32P-ATP, as described previously (see Ferrandi M. et al., Hypertension 1996; 28(6):1018-25). Increasing concentrations of the standard ouabain, or tested compound, were incubated with 0.3 μg of purified dog kidney enzyme for 10 min at 37° C. in 120 μl final volume of a medium containing 140 mM NaCl, 3 mM MgCl2, 50 mM Hepes-Tris, 3 mM ATP at a pH 7.5. Then, 10 μl of incubation solution containing 10 mM KCl and 20 nCi of 32P-ATP (3-10 Ci/mmol, Perkin Elmer) was added, and the reaction was continued for 15 min at 37° C. The reaction was then stopped by acidification with 20% v/v ice-cold perchloric acid. 32P was separated by centrifugation with activated Charcoal (Norit A, Serva) and the radioactivity was measured. The inhibitory activity was expressed as percent of the control samples carried out in the absence of ouabain or tested compound. The concentration of compound causing 50% inhibition of the Na,K-ATPase activity (IC50) was calculated by using a multiple parameter non-linear regression best fitting program (Kaleidagraph™, Sinergy Software).
- Biological Assay The Inhibitory Activity of the Present Compounds on Human ROMK and Rat ROMK ChannelsThe method described hereafter was used for determining the inhibitory activity of the present compounds on human ROMK and rat ROMK channels.1. Materials and Instruments(1) FluxOR potassium ion channel assay (F10016, Invitrogen)(2) Ouabain (O3125-1G, Sigma)(3) FlexStation3 microplate reader (Molecular Devices)(4) Human ROMK/HEK293 cell: HEK293 cell line stably expressing the ROMK channel transfected by human ROMK cDNA (NCBI SEQ ID NO. NM-000220.4)(5) Rat ROMK/HEK293 cell: HEK293 cell line transfected by rat ROMK cDNA (NCBI SEQ ID NO. NM-017023.1) stably expressing the ROMK channel(6) HEK293 cell line: Cell Bank of Chinese Academy of Sciences, GNHu432. Experimental ProcedureExcept for ddH2O and Ouabain, all of the experimental reagents are from FluxOR Potassium Ion Channel Assay Kit and the formulation methods also refer to the kit instructions. (1) Human ROMK/HEK293 cell was seeded on PDL (Poly-D-lysine) coated plates at 20000 cells/well on the previous day; (2) After overnight culture, the plate medium was discarded; then according to the Fluxor Potassium Ion Channel Assay Kit instructions, the dye was added at 100 μL/hole, and then incubated for 90 mins at room temperature; (3) The dye was then decanted and 1004, of assay buffer containing ouabain (30004) and probenecid were added in each well; (4) 1 μL of compound or DMSO was added to the corresponding wells, shocked for 30 seconds, and incubated for 30 mins at room temperature; (5) The plates were placed in a FlexStation3 microplate reader, and then added with stimulation buffer (K2SO4: Tl2SO4: 1×FluxOR Chloride-free Buffer: ddH2O=3:12:40:125) at 25 μL/well, then the value was read continuously for 5 mins at EX/EM of 490/525 nm immediately; and (6) The IC50 of the present compounds on human ROMK channel was obtained by data processing software Graphpad.
- Inhibitory Activity Assay human ROMK and rat ROMK: 1. Materials and Instruments (1) FluxOR potassium ion channel assay (F10016, Invitrogen) (2) Ouabain (03125-1G, Sigma) (3) FlexStation3 microplate reader (Molecular Devices) (4) Human ROMK/HEK293 cell: HEK293 cell line stably expressing the ROMK channel transfected by human ROMK cDNA (NCBI SEQ ID NO. NM-000220.4) (5) Rat ROMK/HEK293 cell: HEK293 cell line transfected by rat ROMK cDNA (NCBI SEQ ID NO. NM-017023.1) stably expressing the ROMK channel (6) HEK293 cell line: Cell Bank of Chinese Academy of Sciences, GNHu43 2. Experimental Procedure - Human ROMK/HEK293 cell was seeded on PDL(Poly-D-lysine) coated plates at 20000 cells/well on the previous day; After overnight culture, the plate medium was discarded; then according to the Fluxor Potassium Ion Channel Assay Kit instructions, the dye was added at 100 μL/hole, and then incubated for 90 mins at room temperature; The dye was then decanted and 100 μL of assay buffer containing ouabain (300 μM) and probenecid were added in each well;1 μL of compound or DMSO was added to the corresponding wells, shocked for 30 seconds, and incubated for 30 mins at room temperature; The plates were placed in a FlexStation3 microplate reader, and then added with stimulation buffer (K2SO4:Tl2SO4:1XFluxOR Chloride-free Buffer:ddH2O=3:12:40:125) at 25 μL/well, then the value was read continuously for 5 mins at EX/EM of 490/525 nm immediately; and The IC50 of the present compounds on human ROMK channel was obtained by data processing software Graphpad.
- Inhibition Assay Human HEK293 cells over-expressing human IK are grown in culture medium (DMEM supplemented with 10% foetal bovine serum), in polystyrene culture flasks (175 mm2) in a humidified atmosphere of 5% CO2 in air, at 37° C. Cell confluence should be 80-90% on day of plating. Cells are rinsed with 4 mL of PBS (phosphate buffered saline) and incubated 2 min with 1 mL of Trypsin-EDTA. After addition of 25 mL of culture medium cells are re-suspended by trituration with a 25 mL pipette.The cells are seeded at a density of 3×106 cells/mL (25 μL/well) in black-walled, clear bottom, 384-well plates pre-treated with 0.01 g/L poly-D-lysin (20 μL/well for ≧30 min). Plated cells were allowed to proliferate for 24 h before loading with dye.BTC-AM (50 mg, Invitrogen) is added 25.5 μl DMSO. The BTC-AM stock solution (2 mM) is diluted to a final concentration of 2 μM in Cl+ free assay buffer (in mM: 140 Na+-gluconate, 2.5 K+-gluconate, 6 Ca2+-gluconate, 1 Mg2+ gluconate, 5 glucose, 10 HEPES, pH 7.3) containing 2 μM ouabain, 2 mM amaranth and 1 mM tartrazine.The culture medium is aspirated from the wells, and 25 μl of the BTC-AM loading solution are added to each well. The cells are incubated at 37° C. for 60 min.After the loading period, the Tl+-sensitive BTC fluorescence signal is measured over time using a FLIPR.
- Selective Inhibition Assays of Isolated Na,K-ATPase To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isoform complexes α1β1FXYD1, α2β1FXYD1, α2β2FXTD1 and α2β3FXYD1. Although all the preparations and assays were conducted with FXYD1 in order to stabilize the complexes, the FXYD1 suffix is omitted in naming of isoform complexes for simplicity.Na,K-ATPase activity of α/βPFXYD1 complexes was measured over one hour at 37° C. in a medium containing 130 mM NaCl, 5 mM KCl, 3 mM MgCl2, 1 mM EGTA, 25 mM Histidine, pH 7.4 and 1 mM ATP using the PiColor Lock gold malachite green assay (Inova Biosciences).The Na,K-ATPase activities were α1β1, 21.5±5.3 μmoles/min/mg; α2β1, 18.7±1.8 μmoles/min/mg, and α2β3, 10.7±1.9 μmoles/min/mg protein. As discussed below, an important kinetic property in relation to inhibition by cardiac glycosides is K0.5 for activation by K: α1β1-1.25±0.05 mM, α2β1-2.7±0.14 mM and α2β3 6.4±0.50 mM, respectively.Selectivity of the compounds for various isolated isoforms of human Na,K-ATPase was determined essentially as described before [Katz, A. et al., J Biol Chem., 2010, 285(25), pp. 19582-19592].ATPase activity assays as well as titrations with NaCl, KCl and vanadate were performed as described in Lifshitz-2007 and Loayza-1998 using PiColorLock malachite green assay (Inova Bioscience). Inhibitor assays were performed as described in Katz-2010. [3H]ouabain binding and K+-[3H]digoxin displacement assays were performed as described in Katz-2010.The percent inhibition VCG/V0 was calculated and Ki values were obtained by fitting the data to the function VCG/V0=Ki/([CG]+Ki)+c (CG stands for cardiac glycoside). Inhibition was estimated in 3-5 separate experiments and average Ki values±standard error of the mean (SEM) were calculated. The ratios Ki α1β1/α2β1, α1β1/α2β2 and α1β1/α2β3 was calculated for each compound.
- Thallium Flux Assay Solutions and reagents: Thallium flux assay was performed using FluxOR kit (F10017, Life Technologies). Loading buffer, assay buffer and stimulus buffer were prepared using kit components. HBSS (Hank's balanced salt solution, Cat #14025-092) was purchased separately from Life Technologies.To prepare 10 ml of loading buffer: 10 μl of FluxOR dye (reconstituted in DMSO) was first added to 100 μl of powerload concentrate and this mix along with 100 μl of Probenicid (100×) was then added to 9.79 ml of HBSS. Assay buffer (10 ml) was prepared by addition of 2 ml of FluxOR chloride free buffer (5×), 100 μl of Probenicid (100×), and 0.2 ml of Ouabain (13.77 mM) to 7.7 ml of deionized water. Stimulus buffer was composed of 15 mM Tl2SO4, 0.75 mM K2SO4 in FluxOR chloride free buffer (diluted to 1× using deionized water). The final concentration of Tl2SO4 and K2SO4 in the assay plate was 3 mM and 0.15 mM, respectively.Plating and induction of cells: The CHO T-Rex hROMK (human Kir1.1) stable cell line was maintained in Ham's F12 media supplemented with 10% FBS, 1% Penicillin-Streptomycin, 500 μg/ml Zeocin and 10 μg/ml Blasticidin at 37° C. in a 5% CO2 incubator. One day before the experiment, the cells were dissociated by incubation with Versene solution (15040-066, Life Technologies) for 10 minutes at 37° C. followed by addition of growth media. The cell suspension was centrifuged at 1200 rpm for 5 min. After discarding the supernatant, the cells were resuspended in fresh growth media and cell concentration was determined using a hemocytometer. Next, 0.5 μg/ml of Doxycycline was added to the cell suspension to induce hROMK channel expression and 50 μl (10,000 cells/well) of cell suspension was added to each well of a poly-D lysine coated 384 well black, optically clear bottom plate (6007718, Perkin Elmer). The assay plate was kept at 37° C. in a 5% CO2 incubator.Assay protocol: On the day of experiment, media was removed and loading buffer was added (30 μl/well) to the assay plate. The cells were incubated in the loading buffer for 30 minutes at 37° C. The loading buffer was then replaced by assay buffer (30 μl/well) followed by addition of test compounds or controls. The cells were incubated with compounds for 30 minutes and the plate was then mounted on FlexStation (Molecular Devices) for fluorescence read out with excitation and emission wavelengths at 488 and 525 nm, respectively. Each well was read for 90 sec at 2 sec interval and the stimulus buffer was added after 20 seconds of baseline recording. The final DMSO concentration was either 0.5 or 1% in the assay plate. Positive and negative controls were defined by addition of DMSO or 3 μM of a standard ROMK inhibitor, respectively, to the wells instead of a test compound.Data analysis: The slope (over a period of 15 seconds) of fluorescence increase after stimulus buffer addition was exported from SoftMax Pro into a custom made software where it was converted to % inhibition. A 10-point concentration response curve was used to estimate the IC50 value of test compounds.
 4-((1R,3S,5S,8R,9S,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[(1R,3S,5S,10R,11R,13R,14S,17R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one Ouabain4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one(Ouabain) Ouabain CHEMBL222863 4-[(R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one cid_439501 BDBM50286739 NSC-25485
4-((1R,3S,5S,8R,9S,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[(1R,3S,5S,10R,11R,13R,14S,17R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one Ouabain4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tetrahydroxy-10-(hydroxymethyl)-13-methyl-3-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yloxy)-hexadecahydro-1H-cyclopenta[a]phenanthren-17-yl)furan-2(5H)-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one(Ouabain) Ouabain CHEMBL222863 4-[(R)-1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one 4-[1,5,11,14-Tetrahydroxy-10-hydroxymethyl-13-methyl-3-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-hexadecahydro-cyclopenta[a]phenanthren-17-yl]-5H-furan-2-one cid_439501 BDBM50286739 NSC-25485