Target (2)
Compound (31)
Article Title (46)
Assay (24)
Bonazzi, S; Connolly, M; Glass, DJ; Mihalic, M; Patterson, AW; Roggo, S; Shavlakadze, T Rapamycin derivatives US Patent US12091424 (2024) BONAZZI, S; CONNOLLY, M; GLASS, DJ; MIHALIC, M; PATTERSON, AW; ROGGO, S; SHAVLAKADZE, T NOVEL RAPAMYCIN DERIVATIVES US Patent US20250223297 (2025) Wagner, R; Mollison, KW; Liu, L; Henry, CL; Rosenberg, TA; Bamaung, N; Tu, N; Wiedeman, PE; Or, Y; Luly, JR; Lane, BC; Trevillyan, J; Chen, YW; Fey, T; Hsieh, G; Marsh, K; Nuss, M; Jacobson, PB; Wilcox, D; Carlson, RP; Carter, GW; Djuric, SW Rapamycin analogs with reduced systemic exposure. Bioorg Med Chem Lett 15: 5340 -3 (2005) Gregory, MA; Kendrew, SG; Moss, SJ; Wilkinson, B Rapamycin analogues and their pharmaceutical use US Patent US9505773 (2016) Edwards, SR; Wandless, TJ The rapamycin-binding domain of the protein kinase mammalian target of rapamycin is a destabilizing domain. J Biol Chem 282: 13395 -401 (2007) Dickman, DA; Ding, H; Li, Q; Nilius, AM; Balli, DJ; Ballaron, SJ; Trevillyan, JM; Smith, ML; Seif, LS; Kim, K; Sarthy, A; Goldman, RC; Plattner, JJ; Bennani, YL Antifungal rapamycin analogues with reduced immunosuppressive activity. Bioorg Med Chem Lett 10: 1405 -8 (2000) Abdel-Magid, AF Rapalogs Potential as Practical Alternatives to Rapamycin. ACS Med Chem Lett 10: 843 -845 (2019) Mortensen, DS; Perrin-Ninkovic, SM; Shevlin, G; Zhao, J; Packard, G; Bahmanyar, S; Correa, M; Elsner, J; Harris, R; Lee, BG; Papa, P; Parnes, JS; Riggs, JR; Sapienza, J; Tehrani, L; Whitefield, B; Apuy, J; Bisonette, RR; Gamez, JC; Hickman, M; Khambatta, G; Leisten, J; Peng, SX; Richardson, SJ; Cathers, BE; Canan, SS; Moghaddam, MF; Raymon, HK; Worland, P; Narla, RK; Fultz, KE; Sankar, S Discovery of mammalian target of rapamycin (mTOR) kinase inhibitor CC-223. J Med Chem 58: 5323 -33 (2015) Sedrani, R; Jones, LH; Jutzi-Eme, AM; Schuler, W; Cottens, S Cleavage of the cyclohexyl-subunit of rapamycin results in loss of immunosuppressive activity. Bioorg Med Chem Lett 9: 459 -62 (1999) Zask, A; Kaplan, J; Verheijen, JC; Richard, DJ; Curran, K; Brooijmans, N; Bennett, EM; Toral-Barza, L; Hollander, I; Ayral-Kaloustian, S; Yu, K Morpholine derivatives greatly enhance the selectivity of mammalian target of rapamycin (mTOR) inhibitors. J Med Chem 52: 7942 -5 (2009) Nowak, P; Cole, DC; Brooijmans, N; Bursavich, MG; Curran, KJ; Ellingboe, JW; Gibbons, JJ; Hollander, I; Hu, Y; Kaplan, J; Malwitz, DJ; Toral-Barza, L; Verheijen, JC; Zask, A; Zhang, WG; Yu, K Discovery of potent and selective inhibitors of the mammalian target of rapamycin (mTOR) kinase. J Med Chem 52: 7081 -9 (2009) Knight, SD; Adams, ND; Burgess, JL; Chaudhari, AM; Darcy, MG; Donatelli, CA; Luengo, JI; Newlander, KA; Parrish, CA; Ridgers, LH; Sarpong, MA; Schmidt, SJ; Van Aller, GS; Carson, JD; Diamond, MA; Elkins, PA; Gardiner, CM; Garver, E; Gilbert, SA; Gontarek, RR; Jackson, JR; Kershner, KL; Luo, L; Raha, K; Sherk, CS; Sung, CM; Sutton, D; Tummino, PJ; Wegrzyn, RJ; Auger, KR; Dhanak, D Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett 1: 39 -43 Park, H; Choe, H; Hong, S Virtual screening and biochemical evaluation to identify new inhibitors of mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 24: 835 -8 (2014) Venkatesan, AM; Chen, Z; dos Santos, O; Dehnhardt, C; Santos, ED; Ayral-Kaloustian, S; Mallon, R; Hollander, I; Feldberg, L; Lucas, J; Yu, K; Chaudhary, I; Mansour, TS PKI-179: an orally efficacious dual phosphatidylinositol-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitor. Bioorg Med Chem Lett 20: 5869 -73 (2010) Venkateswarlu, V; Pathania, AS; Aravinda Kumar, KA; Mahajan, P; Nargotra, A; Vishwakarma, RA; Malik, FA; Sawant, SD 4-(N-Phenyl-N'-substituted benzenesulfonyl)-6-(4-hydroxyphenyl)quinolines as inhibitors of mammalian target of rapamycin. Bioorg Med Chem 23: 4237 -47 (2015) Estrada, AA; Shore, DG; Blackwood, E; Chen, YH; Deshmukh, G; Ding, X; Dipasquale, AG; Epler, JA; Friedman, LS; Koehler, MF; Liu, L; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Sideris, S; St-Jean, F; Trinh, L; Truong, T; Lyssikatos, JP Pyrimidoaminotropanes as potent, selective, and efficacious small molecule kinase inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem 56: 3090 -101 (2013) Zask, A; Verheijen, JC; Curran, K; Kaplan, J; Richard, DJ; Nowak, P; Malwitz, DJ; Brooijmans, N; Bard, J; Svenson, K; Lucas, J; Toral-Barza, L; Zhang, WG; Hollander, I; Gibbons, JJ; Abraham, RT; Ayral-Kaloustian, S; Mansour, TS; Yu, K ATP-Competitive Inhibitors of the Mammalian Target of Rapamycin: Design and Synthesis of Highly Potent and Selective Pyrazolopyrimidines J Med Chem 52: 5013 -6 (2009) Bonazzi, S; Goold, CP; Gray, A; Thomsen, NM; Nunez, J; Karki, RG; Gorde, A; Biag, JD; Malik, HA; Sun, Y; Liang, G; Lubicka, D; Salas, S; Labbe-Giguere, N; Keaney, EP; McTighe, S; Liu, S; Deng, L; Piizzi, G; Lombardo, F; Burdette, D; Dodart, JC; Wilson, CJ; Peukert, S; Curtis, D; Hamann, LG; Murphy, LO Discovery of a Brain-Penetrant ATP-Competitive Inhibitor of the Mechanistic Target of Rapamycin (mTOR) for CNS Disorders. J Med Chem 63: 1068 -1083 (2020) Ballou, LM; Selinger, ES; Choi, JY; Drueckhammer, DG; Lin, RZ Inhibition of mammalian target of rapamycin signaling by 2-(morpholin-1-yl)pyrimido[2,1-alpha]isoquinolin-4-one. J Biol Chem 282: 24463 -70 (2007) Tao, Z; Barker, J; Shi, SD; Gehring, M; Sun, S Steady-state kinetic and inhibition studies of the mammalian target of rapamycin (mTOR) kinase domain and mTOR complexes. Biochemistry 49: 8488 -98 (2010) D'Angelo, ND; Kim, TS; Andrews, K; Booker, SK; Caenepeel, S; Chen, K; D'Amico, D; Freeman, D; Jiang, J; Liu, L; McCarter, JD; San Miguel, T; Mullady, EL; Schrag, M; Subramanian, R; Tang, J; Wahl, RC; Wang, L; Whittington, DA; Wu, T; Xi, N; Xu, Y; Yakowec, P; Yang, K; Zalameda, LP; Zhang, N; Hughes, P; Norman, MH Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem 54: 1789 -811 (2011) Yoon, S; Kim, JH; Kim, SE; Kim, C; Tran, PT; Ann, J; Koh, Y; Jang, J; Kim, S; Moon, HS; Kim, WK; Lee, S; Lee, J; Kim, S; Lee, J Discovery of Leucyladenylate Sulfamates as Novel Leucyl-tRNA Synthetase (LRS)-Targeted Mammalian Target of Rapamycin Complex 1 (mTORC1) Inhibitors. J Med Chem 59: 10322 -10328 (2016) Cohen, F; Bergeron, P; Blackwood, E; Bowman, KK; Chen, H; Dipasquale, AG; Epler, JA; Koehler, MF; Lau, K; Lewis, C; Liu, L; Ly, CQ; Malek, S; Nonomiya, J; Ortwine, DF; Pei, Z; Robarge, KD; Sideris, S; Trinh, L; Truong, T; Wu, J; Zhao, X; Lyssikatos, JP Potent, selective, and orally bioavailable inhibitors of mammalian target of rapamycin (mTOR) kinase based on a quaternary substituted dihydrofuropyrimidine. J Med Chem 54: 3426 -35 (2011) Koehler, MF; Bergeron, P; Blackwood, E; Bowman, KK; Chen, YH; Deshmukh, G; Ding, X; Epler, J; Lau, K; Lee, L; Liu, L; Ly, C; Malek, S; Nonomiya, J; Oeh, J; Ortwine, DF; Sampath, D; Sideris, S; Trinh, L; Truong, T; Wu, J; Pei, Z; Lyssikatos, JP Potent, selective, and orally bioavailable inhibitors of the mammalian target of rapamycin kinase domain exhibiting single agent antiproliferative activity. J Med Chem 55: 10958 -71 (2012) Richard, DJ; Verheijen, JC; Yu, K; Zask, A Triazines incorporating (R)-3-methylmorpholine are potent inhibitors of the mammalian target of rapamycin (mTOR) with selectivity over PI3Kalpha. Bioorg Med Chem Lett 20: 2654 -7 (2010) Chen, Y; Yuan, X; Zhang, W; Tang, M; Zheng, L; Wang, F; Yan, W; Yang, S; Wei, Y; He, J; Chen, L Discovery of Novel Dual Histone Deacetylase and Mammalian Target of Rapamycin Target Inhibitors as a Promising Strategy for Cancer Therapy. J Med Chem 62: 1577 -1592 (2019) Yoon, S; Kim, JH; Koh, Y; Tran, PT; Ann, J; Yoon, I; Jang, J; Kim, WK; Lee, S; Lee, J; Kim, S; Lee, J Discovery of simplified leucyladenylate sulfamates as novel leucyl-tRNA synthetase (LRS)-targeted mammalian target of rapamycin complex 1 (mTORC1) inhibitors. Bioorg Med Chem 25: 4145 -4152 (2017) Bursavich, MG; Brooijmans, N; Feldberg, L; Hollander, I; Kim, S; Lombardi, S; Park, K; Mallon, R; Gilbert, AM Novel benzofuran-3-one indole inhibitors of PI3 kinase-alpha and the mammalian target of rapamycin: hit to lead studies. Bioorg Med Chem Lett 20: 2586 -90 (2010) Mortensen, DS; Perrin-Ninkovic, SM; Shevlin, G; Elsner, J; Zhao, J; Whitefield, B; Tehrani, L; Sapienza, J; Riggs, JR; Parnes, JS; Papa, P; Packard, G; Lee, BG; Harris, R; Correa, M; Bahmanyar, S; Richardson, SJ; Peng, SX; Leisten, J; Khambatta, G; Hickman, M; Gamez, JC; Bisonette, RR; Apuy, J; Cathers, BE; Canan, SS; Moghaddam, MF; Raymon, HK; Worland, P; Narla, RK; Fultz, KE; Sankar, S Optimization of a Series of Triazole Containing Mammalian Target of Rapamycin (mTOR) Kinase Inhibitors and the Discovery of CC-115. J Med Chem 58: 5599 -608 (2015) Curran, KJ; Verheijen, JC; Kaplan, J; Richard, DJ; Toral-Barza, L; Hollander, I; Lucas, J; Ayral-Kaloustian, S; Yu, K; Zask, A Pyrazolopyrimidines as highly potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 1-substituent. Bioorg Med Chem Lett 20: 1440 -4 (2010) Tsou, HR; MacEwan, G; Birnberg, G; Zhang, N; Brooijmans, N; Toral-Barza, L; Hollander, I; Ayral-Kaloustian, S; Yu, K 4-Substituted-7-azaindoles bearing a ureidobenzofuranone moiety as potent and selective, ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20: 2259 -63 (2010) Takeuchi, CS; Kim, BG; Blazey, CM; Ma, S; Johnson, HW; Anand, NK; Arcalas, A; Baik, TG; Buhr, CA; Cannoy, J; Epshteyn, S; Joshi, A; Lara, K; Lee, MS; Wang, L; Leahy, JW; Nuss, JM; Aay, N; Aoyama, R; Foster, P; Lee, J; Lehoux, I; Munagala, N; Plonowski, A; Rajan, S; Woolfrey, J; Yamaguchi, K; Lamb, P; Miller, N Discovery of a novel class of highly potent, selective, ATP-competitive, and orally bioavailable inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem 56: 2218 -34 (2013) Dehnhardt, CM; Venkatesan, AM; Delos Santos, E; Chen, Z; Santos, O; Ayral-Kaloustian, S; Brooijmans, N; Mallon, R; Hollander, I; Feldberg, L; Lucas, J; Chaudhary, I; Yu, K; Gibbons, J; Abraham, R; Mansour, TS Lead optimization of N-3-substituted 7-morpholinotriazolopyrimidines as dual phosphoinositide 3-kinase/mammalian target of rapamycin inhibitors: discovery of PKI-402. J Med Chem 53: 798 -810 (2010) Castro-Falcón, G; Seiler, GS; Demir, Ö; Rathinaswamy, MK; Hamelin, D; Hoffmann, RM; Makowski, SL; Letzel, AC; Field, SJ; Burke, JE; Amaro, RE; Hughes, CC Neolymphostin A Is a Covalent Phosphoinositide 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Dual Inhibitor That Employs an Unusual Electrophilic Vinylogous Ester. J Med Chem 61: 10463 -10472 (2018) Stec, MM; Andrews, KL; Bo, Y; Caenepeel, S; Liao, H; McCarter, J; Mullady, EL; San Miguel, T; Subramanian, R; Tamayo, N; Whittington, DA; Wang, L; Wu, T; Zalameda, LP; Zhang, N; Hughes, PE; Norman, MH The imidazo[1,2-a]pyridine ring system as a scaffold for potent dual phosphoinositide-3-kinase (PI3K)/mammalian target of rapamycin (mTOR) inhibitors. Bioorg Med Chem Lett 25: 4136 -42 (2015) Pan, Z; Chen, Y; Pang, H; Wang, X; Zhang, Y; Xie, X; He, G Design, synthesis, and biological evaluation of novel dual inhibitors of heat shock protein 90/mammalian target of rapamycin (Hsp90/mTOR) against bladder cancer cells. Eur J Med Chem 242: (2022) Nishimura, N; Siegmund, A; Liu, L; Yang, K; Bryan, MC; Andrews, KL; Bo, Y; Booker, SK; Caenepeel, S; Freeman, D; Liao, H; McCarter, J; Mullady, EL; San Miguel, T; Subramanian, R; Tamayo, N; Wang, L; Whittington, DA; Zalameda, L; Zhang, N; Hughes, PE; Norman, MH Phospshoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors: discovery and structure-activity relationships of a series of quinoline and quinoxaline derivatives. J Med Chem 54: 4735 -51 (2011) Stec, MM; Andrews, KL; Booker, SK; Caenepeel, S; Freeman, DJ; Jiang, J; Liao, H; McCarter, J; Mullady, EL; San Miguel, T; Subramanian, R; Tamayo, N; Wang, L; Yang, K; Zalameda, LP; Zhang, N; Hughes, PE; Norman, MH Structure-activity relationships of phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors: investigations of various 6,5-heterocycles to improve metabolic stability. J Med Chem 54: 5174 -84 (2011) Luengo, JI; Yamashita, DS; Dunnington, D; Beck, AK; Rozamus, LW; Yen, HK; Bossard, MJ; Levy, MA; Hand, A; Newman-Tarr, T Structure-activity studies of rapamycin analogs: evidence that the C-7 methoxy group is part of the effector domain and positioned at the FKBP12-FRAP interface. Chem Biol 2: 471 -81 (1995) Sutherlin, DP; Bao, L; Berry, M; Castanedo, G; Chuckowree, I; Dotson, J; Folks, A; Friedman, L; Goldsmith, R; Gunzner, J; Heffron, T; Lesnick, J; Lewis, C; Mathieu, S; Murray, J; Nonomiya, J; Pang, J; Pegg, N; Prior, WW; Rouge, L; Salphati, L; Sampath, D; Tian, Q; Tsui, V; Wan, NC; Wang, S; Wei, B; Wiesmann, C; Wu, P; Zhu, BY; Olivero, A Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J Med Chem 54: 7579 -87 (2011) Venkatesan, AM; Dehnhardt, CM; Delos Santos, E; Chen, Z; Dos Santos, O; Ayral-Kaloustian, S; Khafizova, G; Brooijmans, N; Mallon, R; Hollander, I; Feldberg, L; Lucas, J; Yu, K; Gibbons, J; Abraham, RT; Chaudhary, I; Mansour, TS Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5'-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem 53: 2636 -45 (2010) Verheijen, JC; Richard, DJ; Curran, K; Kaplan, J; Lefever, M; Nowak, P; Malwitz, DJ; Brooijmans, N; Toral-Barza, L; Zhang, WG; Lucas, J; Hollander, I; Ayral-Kaloustian, S; Mansour, TS; Yu, K; Zask, A Discovery of 4-morpholino-6-aryl-1H-pyrazolo[3,4-d]pyrimidines as highly potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR): optimization of the 6-aryl substituent. J Med Chem 52: 8010 -24 (2009) Tsou, HR; MacEwan, G; Birnberg, G; Grosu, G; Bursavich, MG; Bard, J; Brooijmans, N; Toral-Barza, L; Hollander, I; Mansour, TS; Ayral-Kaloustian, S; Yu, K Discovery and optimization of 2-(4-substituted-pyrrolo[2,3-b]pyridin-3-yl)methylene-4-hydroxybenzofuran-3(2H)-ones as potent and selective ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20: 2321 -5 (2010) Kaplan, J; Verheijen, JC; Brooijmans, N; Toral-Barza, L; Hollander, I; Yu, K; Zask, A Discovery of 3,6-dihydro-2H-pyran as a morpholine replacement in 6-aryl-1H-pyrazolo[3,4-d]pyrimidines and 2-arylthieno[3,2-d]pyrimidines: ATP-competitive inhibitors of the mammalian target of rapamycin (mTOR). Bioorg Med Chem Lett 20: 640 -3 (2010) Liu, Q; Wang, J; Kang, SA; Thoreen, CC; Hur, W; Ahmed, T; Sabatini, DM; Gray, NS Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem 54: 1473 -80 (2011) Liu, Q; Chang, JW; Wang, J; Kang, SA; Thoreen, CC; Markhard, A; Hur, W; Zhang, J; Sim, T; Sabatini, DM; Gray, NS Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 53: 7146 -55 (2010)
FKBP12 Assay FKBP12 assay using rapamycin analogs. ChEMBL_305479 (CHEMBL830270) Inhibition of Mammalian target of Rapamycin mTOR ChEMBL_1989567 (CHEMBL4623302) Inhibition of Vps34 in human MCF7-LC3 cells assessed as rapamycin-induced autophagy ChEBML_66293 The compound was tested for binding affinity to FK506 binding protein 12 using Rapamycin as control, with an ascomycin conjugate of alkaline phosphatase in a competition binding assay ChEMBL_66293 (CHEMBL678815) The compound was tested for binding affinity to FK506 binding protein 12 using Rapamycin as control, with an ascomycin conjugate of alkaline phosphatase in a competition binding assay ChEMBL_2080981 (CHEMBL4736772) Binding affinity to FKBP12 (unknown origin) expressed in HEK293 cells co-expressing FRB assessed as inhibition of rapamycin-induced FKBP12-FRB dimerization measured after 25 mins by nano-glo live cell reagent based luminescence assay Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically AGP1 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically CIT2 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although th Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically LAP4 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically MEP2 University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t Confirmatory Cherry Pick 3 SAR Dose Response Multiplex in TOR pathway GFP-fusion proteins for Saccharomyes cerevisiae, specifically RPL19A University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae Assay Provider: Maggie Werner-Washburne, UNM Screening Center/ PI: UNMCMD/ Larry Sklar Lead Biologist: Jun Chen Chemistry Center/ PI: University of Kansas Specialized Chemistry Center/ Jeff Aube Chemistry Center Lead: Jennifer Golden, Blake Peterson Assay Implementation: Jun Chen, Stephanie Sedillo, Anna Waller, Annette Evangelisti, Cristian Bologa, Oleg Ursu, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although t mTOR Assay Mammalian target of rapamycin (mTOR) was assayed by monitoring phosphorylation of GFP-4EBP using a homogeneous time-resolved fluorescence resonance energy transfer format. The mTOR-mediated phosphorylation was measured under initial rate conditions. After incubation, the phosphorylated substrate was detected with Tb-anti-p4E-BP1 antibody before reading on a Perkin-Elmer EnVision Fluorescence Reader (exc 340; em 495/520). Duplicate dose-response curves were fit to an equation of competitive tight-binding inhibition. Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically AGP1 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically CIT2 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically LAP4 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically MEP2 based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth Dose Response of TOR pathway GFP-fusion proteins in Saccharomyes cerevisiae specifically RPL19A based on MLPCN hits University of New Mexico Assay Overview: Assay Support: 1R03 MH086450-01 Project Title: Chemical Screen of TOR pathway GFP fusion proteins in S. cerevisiae PI: Maggie Werner-Washburn Center PI: Larry Sklar Assay Implementation: Jun Chen, Chris Allen, Susan Young, Anna Waller, Mark Carter Assay Background and Significance: The target of rapamycin, TOR, is a ser/thr protein kinase evolutionarily conserved from yeast to man [Wullschleger, et al. 2006]. TOR functions in two distinct protein complexes, TOR complex 1 (TORC1) and TORC2 [Cafferkey, et al. 1993; Stan, et al. 1994]. Curiously, only TOR in TORC1 is bound and inhibited by the lipophilic macrolide rapamycin [Kunz, et al. 1993; Helliwell, et al. 1998; Zhang, et al. 2006]. Although the signaling events up- and downstream of TORC2 (which regulates spatial aspects of growth) have yet to be elucidated in detail, it is well established that TORC1 is a central hub of a signaling network that couples cues from hormones and growth In Vitro Inhibition Assay The Invitrogen (Carlsbad, Calif.) mammalian target of rapamycin (mTOR) Lanthascreen assay can be used to quantitate mTOR kinase activity in an in vitro setting. Active mTOR phosphorylates eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) on residue threonine 46. This phosphorylation event can be detected with a phospho-specific terbium (Tb) labeled Ab, in turn bringing the Tb label in close proximity to the GFP tagged 4E-BP1 and allowing for time-resolved fluorescence resonance energy transfer (TR-FRET), which correlates 4E-BP1 phosphorylation levels with mTOR kinase activity. In Vitro mTOR Assay The Invitrogen (Carlsbad, Calif.) mammalian target of rapamycin (mTOR) Lanthascreen assay can be used to quantitate mTOR kinase activity in an in vitro setting. Active mTOR phosphorylates eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) on residue threonine 46. This phosphorylation event can be detected with a phospho-specific terbium (Tb) labeled Ab, in turn bringing the Tb label in close proximity to the GFP tagged 4E-BP 1 and allowing for time-resolved fluorescence resonance energy transfer (TR-FRET), which correlates 4E-BP1 phosphorylation levels with mTOR kinase activity. PI3K Inhibition Assay PI3K inhibition assay (PI3K Assay (Emmanuelle M, Huang Y, Yan H G et al. Targeting Protein Translation in Human Non-Small Cell Lung Cancer via Combined MEK and Mammalian Target of Rapamycin Suppression. Cancer Res 67:(23). (2007).) was carried out by PI3 Kinase activity/inhibitor assay kit, where PI3 kinase reaction was set up in Glutathione-coated strips/plate for inhibitor reaction. Kinase and inhibitors were pre-incubated for 10 minutes prior to the addition of PIP2 substrate. 5 uL of 5x kinase reaction buffer were added in each well followed by the further addition of 5 uL/well of PIP2 substrate. Then distilled H2O was added to each well so as to make up a final volume of 25 uL/well. Incubation was done at rt for 1 hour which was followed by washing the wells 3 times with 200 uL of 1xTBST per well and then 2 times with 200 uL of IX TBS per well. Then 100 uL of the Substrate TMB per well was added and then to keep for colour development in the dark. SPR Assay to Determine Binding Affinity to FKBP51 Biotinylated avi-FKBP51 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 2000 RU, 3 μg/ml biotinylated avi-FKBP51 were injected for 360 sec at a flow rate of 10 μl/min. Rapalogs were diluted in DMSO to 100× working concentration. Each Rapalog was 100-fold diluted in 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20 and a serial dilution prepared (8 concentrations, 3-fold dilutions, 0.5-1000 nM). Rapamycin was used as reference sample (8 concentrations, 3-fold dilutions, 0.5-1000 nM). The compound dilutions were then injected at 100 uL/min for 120 seconds contact time and with 3600 seconds dissociation time with increasing concentrations. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer. Multi-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). SPR Assay to Determine Binding Affinity to FKBP12 Biotinylated avi-FKBP12 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 1000 RU, 2 μg/ml biotinylated avi-FKBP12 were injected for 100 sec at a flow rate of 10 μl/min. Rapalogs were diluted in DMSO to 100× working concentration. Each Rapalog was 100-fold diluted in 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20 and a serial dilution prepared (9 concentrations, 3-fold dilutions, 0.08-500 nM). Rapamycin was used as reference sample (9 concentrations, 3-fold dilutions, 0.02-100 nM). The compound dilutions were then injected at 100 uL/min for 120 seconds contact time in sequence with increasing concentrations. Dissociation was monitored for 3600 seconds. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer. The single-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). SPR Assay to Characterize Ternary Complex Formation with FKBP12 Biotinylated avi-FKBP12 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 100 RU, 0.3 g/ml biotinylated avi-FKBP12 were injected for 80 sec at a flow rate of 10 l/min. Serial dilution of FRB was prepared (12 concentrations, 3-fold dilutions, 0.00011-20 M) and supplemented with 100 nM of rapalog. A-B-A injection mode was used to ensure saturation immobilized FKBP12 with respective rapalog. 100 nM solution of the respective rapalog was injected before FRB injection for 120 sec and during dissociation for 420 sec. The FRB dilutions were then injected 120 seconds contact time with increasing concentrations. Rapamycin was used as reference sample. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer at a flow rate of 30 l/min. The multi-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). In case of fast association and dissociation, steady state affinity analysis following the law of mass action was used to determine the affinity Kd (M). SPR Assay to Characterize Ternary Complex Formation with FKBP51 Biotinylated avi-FKBP51 was immobilized on a streptavidin chip (Cytiva Series S SA) using a Biacore 8K or 8 k+ (Cytiva). To achieve an immobilization level of 200 RU, 0.6 g/ml biotinylated avi-FKBP51 were injected for 150 sec at a flow rate of 10 l/min. Serial dilution of FRB was prepared (12 concentrations, 3-fold dilutions, 0.00011-20 M) and supplemented with 100 nM of rapalog. A-B-A injection mode was used to ensure saturation immobilized FKBP12 with respective rapalog. 100 nM solution of the respective rapalog was injected before FRB injection for 120 sec and during dissociation for 420 sec. The FRB dilutions were then injected 120 seconds contact time with increasing concentrations. Rapamycin was used as reference sample. 50 mM HEPES pH 7.5, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.05% Tween-20, 1% DMSO was used as running buffer at a flow rate of 30 l/min. The multi-cycle kinetics data were fit to a 1:1 binding model to measure the association rate ka (1/Ms), the dissociation rate kd (1/s) and the affinity Kd (M). In case of fast association and dissociation, steady state affinity analysis following the law of mass action was used to determine the affinity Kd (M).
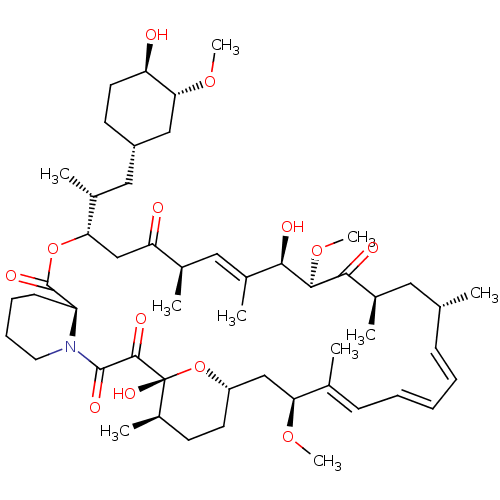 BDBM92863 mTOR Inhibitor, Rapamycin US9505773, Rapamycin
BDBM92863 mTOR Inhibitor, Rapamycin US9505773, Rapamycin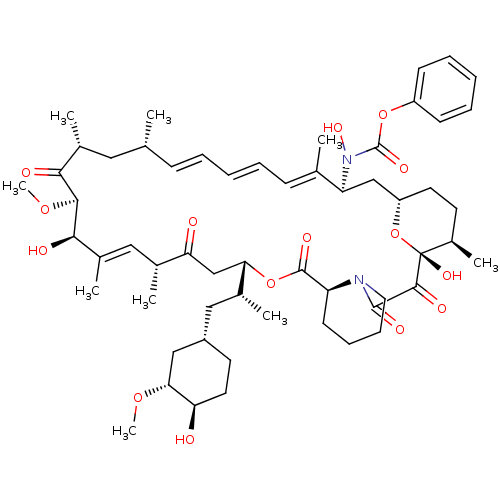 CHEMBL437064 Rapamycin analogue BDBM50089437
CHEMBL437064 Rapamycin analogue BDBM50089437 Rapamycin analogue CHEMBL281297 BDBM50089447
Rapamycin analogue CHEMBL281297 BDBM50089447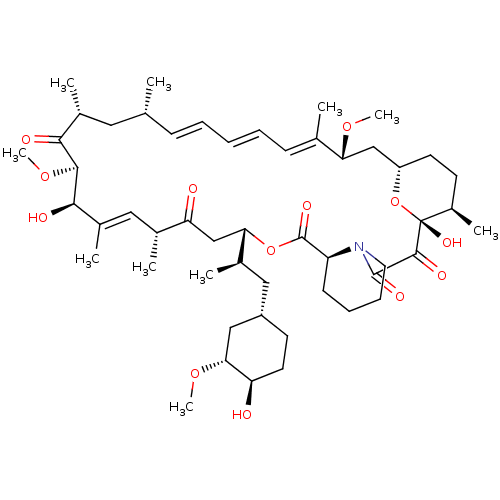 BDBM36608 Rapamycin C-7, analog 1
BDBM36608 Rapamycin C-7, analog 1 BDBM36610 Rapamycin C-7, analog 5a
BDBM36610 Rapamycin C-7, analog 5a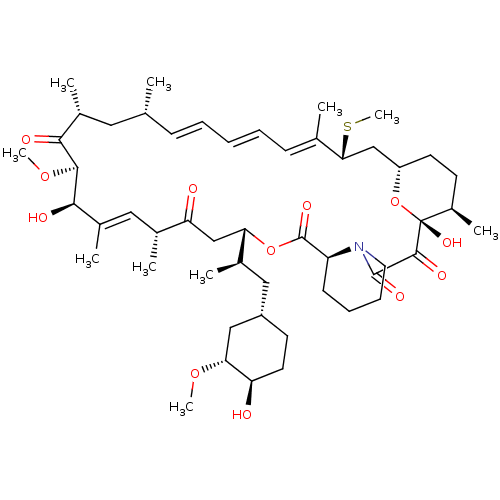 BDBM36612 Rapamycin C-7, analog 6a
BDBM36612 Rapamycin C-7, analog 6a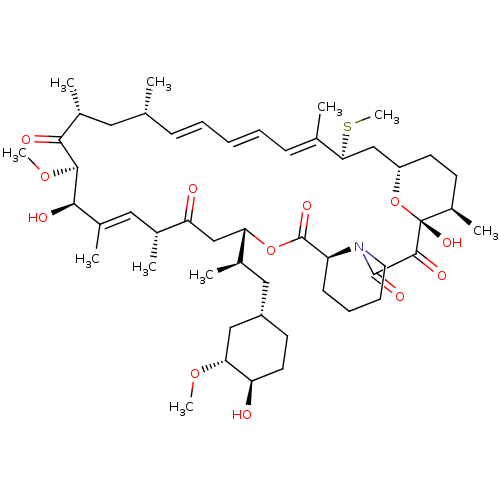 BDBM36613 Rapamycin C-7, analog 6b
BDBM36613 Rapamycin C-7, analog 6b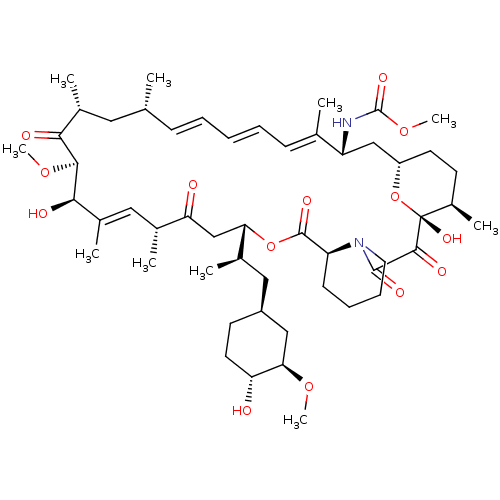 BDBM36614 Rapamycin C-7, analog 7a
BDBM36614 Rapamycin C-7, analog 7a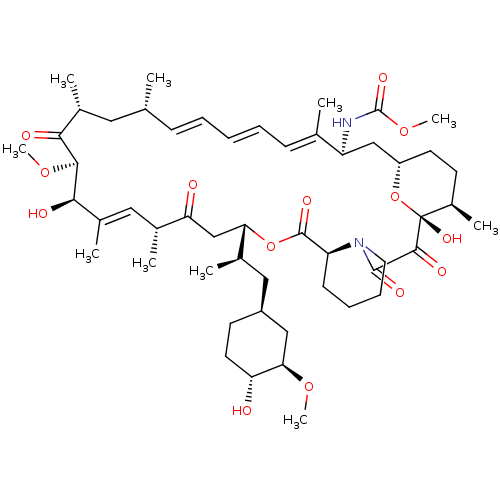 BDBM36615 Rapamycin C-7, analog 7b
BDBM36615 Rapamycin C-7, analog 7b BDBM36616 Rapamycin C-7, analog 8a
BDBM36616 Rapamycin C-7, analog 8a BDBM36617 Rapamycin C-7, analog 8b
BDBM36617 Rapamycin C-7, analog 8b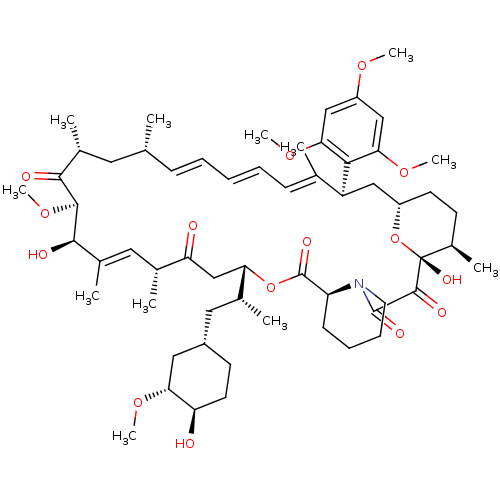 BDBM36618 Rapamycin C-7, analog 9
BDBM36618 Rapamycin C-7, analog 9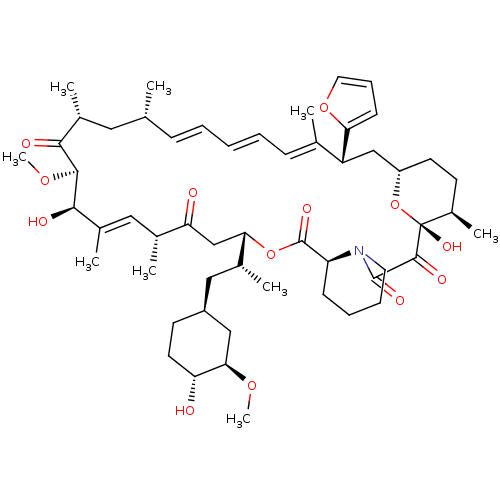 BDBM36619 Rapamycin C-7, analog 10a
BDBM36619 Rapamycin C-7, analog 10a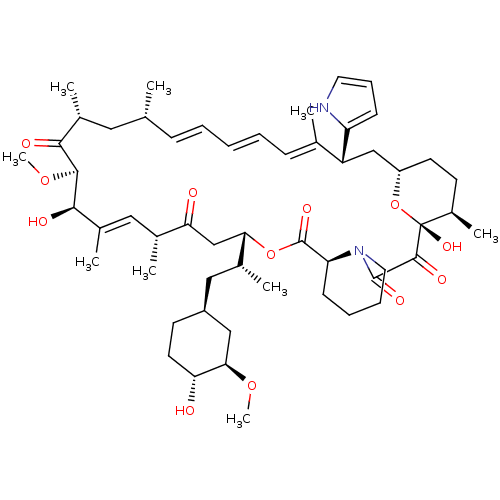 BDBM36621 Rapamycin C-7, analog 11a
BDBM36621 Rapamycin C-7, analog 11a BDBM36622 Rapamycin C-7, analog 11b
BDBM36622 Rapamycin C-7, analog 11b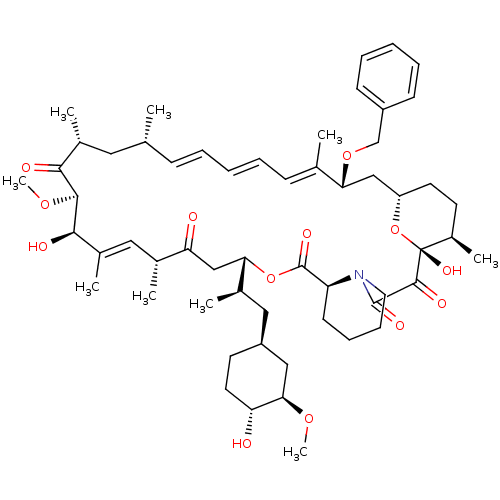 BDBM36625 Rapamycin C-7, analog 14a
BDBM36625 Rapamycin C-7, analog 14a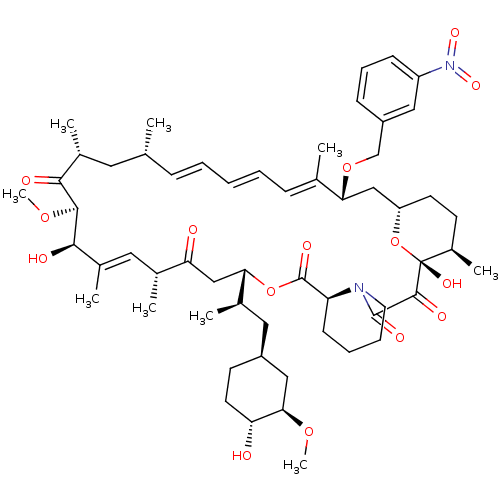 BDBM36626 Rapamycin C-7, analog 15a
BDBM36626 Rapamycin C-7, analog 15a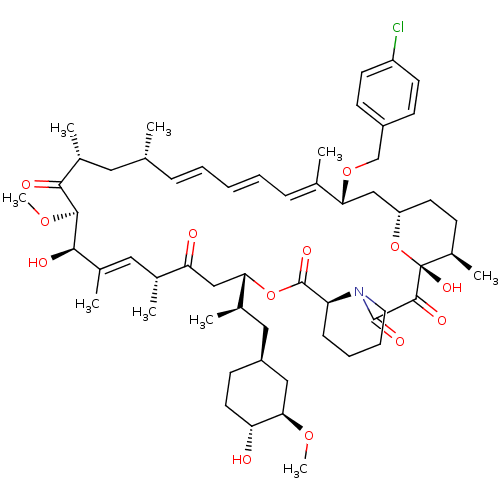 BDBM36627 Rapamycin C-7, analog 16a
BDBM36627 Rapamycin C-7, analog 16a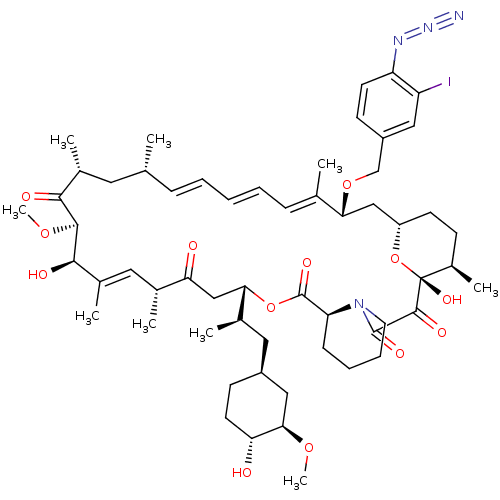 BDBM36628 Rapamycin C-7, analog 17a
BDBM36628 Rapamycin C-7, analog 17a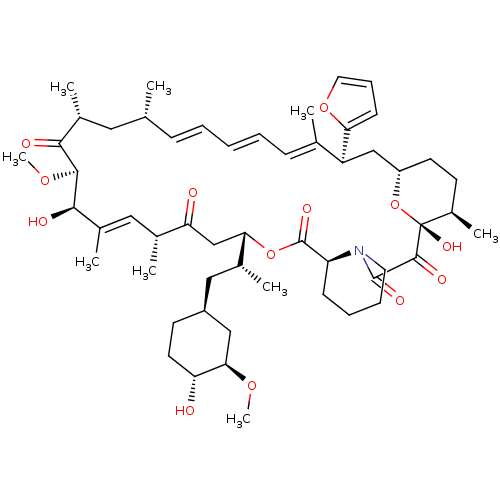 Rapamycin C-7, analog 10b BDBM36620
Rapamycin C-7, analog 10b BDBM36620 Rapamycin C-7, analog 12 BDBM36623
Rapamycin C-7, analog 12 BDBM36623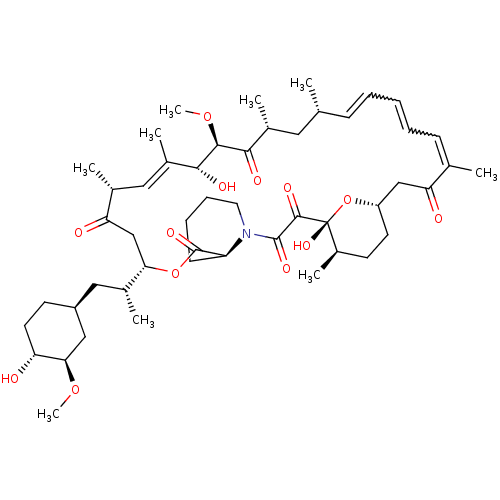 Rapamycin C-7, analog 13 BDBM36624
Rapamycin C-7, analog 13 BDBM36624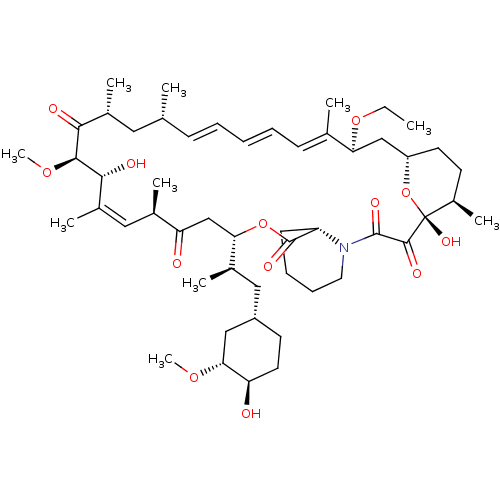 Rapamycin C-7, analog 5b BDBM36611
Rapamycin C-7, analog 5b BDBM36611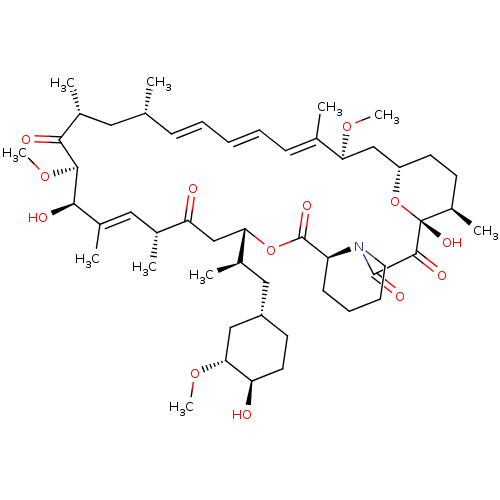 Rapamycin C-7, analog 4 BDBM36609 US11603377, Compound Ramycin SIROLIMUS
Rapamycin C-7, analog 4 BDBM36609 US11603377, Compound Ramycin SIROLIMUS BDBM757304 (S)-C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-rapamycin US20250223297, Example 1
BDBM757304 (S)-C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-rapamycin US20250223297, Example 1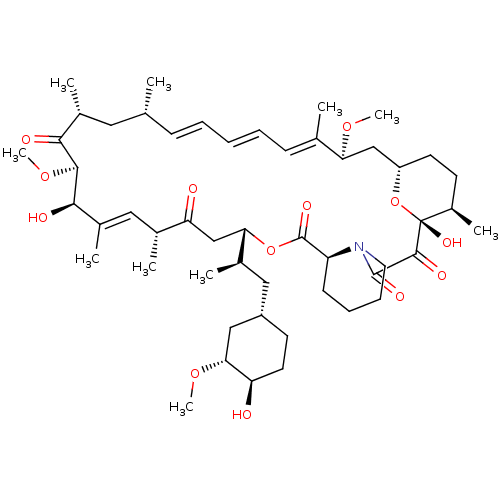 Sirolimus BDBM50064359 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone (Rapamycin) Rapamune Rapamycin AY-22989
Sirolimus BDBM50064359 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone (Rapamycin) Rapamune Rapamycin AY-22989 US12091424, Example 14 C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1) BDBM696419
US12091424, Example 14 C16-(1,1-dioxidoisothiazolidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1) BDBM696419 US12091424, Example 6 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-rapamycin (Diastereomer 1) US20250223297, Example 6 BDBM696416
US12091424, Example 6 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-rapamycin (Diastereomer 1) US20250223297, Example 6 BDBM696416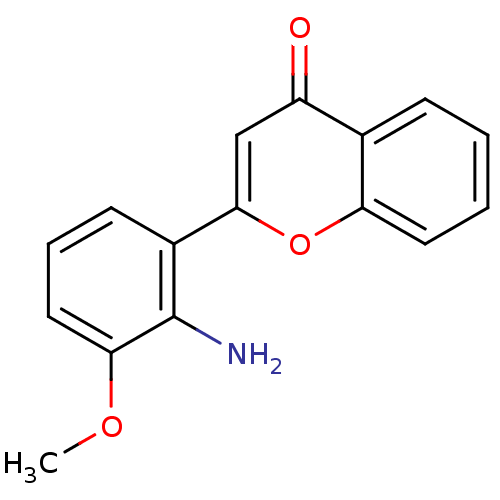 2-(2-amino-3-methoxyphenyl)-4H-chromen-4-one PD-98059 2-(2-Amino-3-methoxy-phenyl)-chromen-4-one(PD98059) BDBM50108771 2-(2-Amino-3-methoxy-phenyl)-chromen-4-one CHEMBL35482 2'-amino-3'-methoxyflavone
2-(2-amino-3-methoxyphenyl)-4H-chromen-4-one PD-98059 2-(2-Amino-3-methoxy-phenyl)-chromen-4-one(PD98059) BDBM50108771 2-(2-Amino-3-methoxy-phenyl)-chromen-4-one CHEMBL35482 2'-amino-3'-methoxyflavone US20250223297, Example 10 US12091424, Example 10 BDBM696418 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1)
US20250223297, Example 10 US12091424, Example 10 BDBM696418 C16-(1,1-dioxido-1,2-thiazetidin-2-yl)-C32-deoxo-C40-dimethylphosphinyl-rapamycin (Diastereomer 1)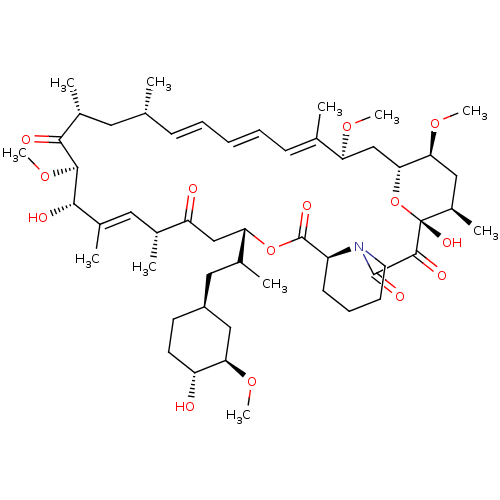 FK-506-M CHEMBL413 Rapamycin (16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2 RAPAMYCIN IMMUNOSUPPRESSANT DRUG BDBM50068561 WY-090217 Rapamune Sirolimus analogue AY-22989 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30,33-trimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone;Rapamycin 1,18-dihydroxy-12-{2-[4-hydroxy-3-methoxy-(4R)-cyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone SIROLIMUS
FK-506-M CHEMBL413 Rapamycin (16E,24E,26E,28E)-1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2 RAPAMYCIN IMMUNOSUPPRESSANT DRUG BDBM50068561 WY-090217 Rapamune Sirolimus analogue AY-22989 1,18-Dihydroxy-12-[2-(4-hydroxy-3-methoxy-cyclohexyl)-1-methyl-ethyl]-19,30,33-trimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-aza-tricyclo[30.3.1.0*4,9*]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone;Rapamycin 1,18-dihydroxy-12-{2-[4-hydroxy-3-methoxy-(4R)-cyclohexyl]-1-methylethyl}-19,30-dimethoxy-15,17,21,23,29,35-hexamethyl-11,36-dioxa-4-azatricyclo[30.3.1.04,9]hexatriaconta-16,24,26,28-tetraene-2,3,10,14,20-pentaone SIROLIMUS