 BDBM512625 PMA
BDBM512625 PMA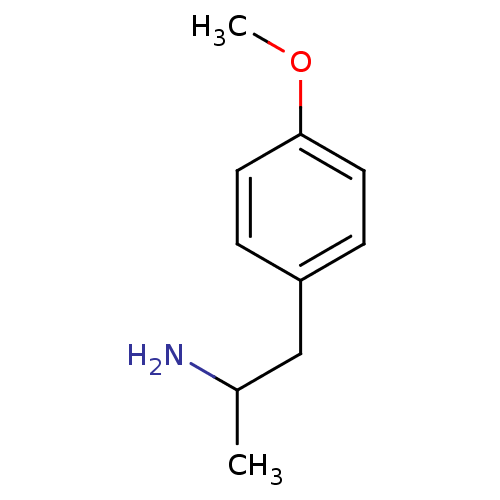 para-Methoxyamphetamine (S)-(+)2-(4-Methoxy-phenyl)-1-methyl-ethylamine (-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine(PMA) (R)-(-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine BDBM50024209 (+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine CHEMBL278663 beta-methoxyamphetamine
para-Methoxyamphetamine (S)-(+)2-(4-Methoxy-phenyl)-1-methyl-ethylamine (-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine(PMA) (R)-(-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine BDBM50024209 (+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine CHEMBL278663 beta-methoxyamphetamine
- ChEMBL_657293 (CHEMBL1249120) Inhibition of AP1 expressed in HEK293 cells assessed as inhibition of PMA-induced transcriptional activation treated 24 hrs before PMA challenge by luciferase reporter gene assay
- ChEMBL_162362 (CHEMBL769169) Effect of PMA on inhibition of PKC alpha (Protein kinase C)
- ChEMBL_2077157 (CHEMBL4732948) Inhibition of PMA-induced human skin fibroblast derived MMP1 by fluorometric assay
- ChEMBL_1712878 (CHEMBL4122927) Inhibition of MALT1 in PMA-stimulated human Jurkat T cells assessed as reduction in IL2 level pretreated for 30 mins followed by PMA-stimulation measured after 5.5 hr by bioluminescence assay
- ChEMBL_625244 (CHEMBL1106187) Inhibition of AP-1 expressed in HEK293 cells assessed as PMA-stimulated transcriptional activation treated 24 hrs before PMA challenge measured after 20 to 24 hrs by luciferase reporter gene assay
- ChEMBL_306871 (CHEMBL828469) In vitro inhibition of PMA-activated human TRPV1 receptor in [Ca2+] influx assay
- ChEMBL_2051225 (CHEMBL4705924) Inhibition of MALT1 in human whole blood assessed as reduction in PMA//anti-CD28 antibody-induced IL2 level pre-incubated for 1 hrs before stimulation with PMA//anti-CD28 antibody by MSD assay
- ChEMBL_2355755 Inhibition of human NOX2 transfected in HEK293T cells assessed as inhibition of PMA-induced superoxide production preincubated for 15 mins followed by PMA stimulation and measured after 10 mins by Amplex-red based analysis
- ChEMBL_2048398 (CHEMBL4703097) Inhibition of PMA-induced MPO in human Neutrophil incubated for 3 mins by luminometry
- ChEMBL_2048423 (CHEMBL4703122) Inhibition of PMA-induced MPO in mouse Neutrophil incubated for 3 mins by luminometry
- ChEMBL_2103824 (CHEMBL4812327) Inhibition of PMA-induced MPO in human Neutrophil incubated for 3 mins by luminometry
- ChEMBL_2355756 Inhibition of NOX2 (unknown origin) expressed in HEK293T cells assessed as inhibition of PMA-induced superoxide production preincubated for 15 mins followed by PMA stimulation and measured after 10 mins by Amplex-red based analysis
- ChEMBL_744549 (CHEMBL1772570) Inhibition of PKC activation in PMA-treated human fibroblasts assessed as inhibition of translocation to plasma membrane cells pretreated for 1 hr followed by 15 mins challenged with PMA by Western blot/densitometric analysis
- ChEMBL_2355728 Inhibition of human NOX1 expressed in CHO cells assessed as inhibition of PMA-induced hydrogen peroxide production pretreated for 15 mins followed by PMA stimulation and measured after 10 mins by Amplex Red based fluorescence analysis
- ChEMBL_2355729 Inhibition of NOX2 in human PLB-985 cells assessed as inhibition of PMA-induced hydrogen peroxide production pretreated for 15 mins followed by PMA stimulation and measured after 10 mins by Amplex Red based fluorescence analysis
- ChEMBL_718965 (CHEMBL1681587) Inhibition of PKD1 autophosphorylation at Ser916 in human PMA-induced LNCAP cells by Western blotting
- ChEMBL_1772743 (CHEMBL4229735) Antagonist activity at CR3 in carboxyfluorescein diacetate succiminidyl ester-labeled human PMN assessed as inhibition of TNF/PMA-stimulated adhesion of PMN to fibrinogen preincubated for 10 mins followed by TNF/PMA addition by fluorescence assay
- ChEMBL_2051224 (CHEMBL4705923) Inhibition of MALT1 in PMA-stimulated human Jurkat T cells assessed as reduction in IL2 level pretreated for 30 mins followed by PMA-stimulation measured after 5.5 hr by human IL-2 promoter driven bioluminescence assay
- ChEMBL_2362820 Inhibition of NOX2 (unknown origin) transfected in COS cells assessed as reduction in PMA-stimulated H2O2 production preincubated for 15 mins followed by PMA stimulation and measured for 1 hr by amplex red dye based fluorescence analysis
- ChEMBL_2501504 Inhibition of NLRP3 inflammasome activation in PMA-differentiated human THP-1 cells assessed as reduction in IL-1beta secretion pre-stimulated with PMA for 24 hrs followed by compound addition and measured after 30 mins by ELISA
- ChEMBL_213814 (CHEMBL819003) In vitro inhibitory potency compared to PMA induced VCAM-1 in human endothelial cells (ELISA assay)
- ChEMBL_2380185 Inhibition of NLRP3 in PMA-differentiated human THP-1 cells assessed as reduction in IL-1beta production
- ChEMBL_650369 (CHEMBL1225085) Inhibition of PMA-stimulated RSK2 phosphorylation in HEK293 cells after 1 hr by Western blotting analysis
- ChEMBL_2024433 (CHEMBL4678246) Allosteric inhibition of MALT1-mediated T cell activation in human Jurkat cells assessed as decrease in PMA induced IL2 activation preincubated for 1 hr followed by addition of PMA measured after 5.5 hrs by luciferase reporter gene assay
- ChEMBL_2355770 Inhibition of NOX1 (unknown origin) expressed in HT 29 cells assessed as inhibition of PMA-induced hydrogen peroxide production pre-incubated for 15 mins and followed by PMA stimulation and measured after 10 mins by Amplex Red based analysis
- ChEMBL_2362821 Inhibition of NOX5 (unknown origin) transfected in HEK cells assessed as reduction in PMA/ionomycin-stimulated H2O2 production preincubated for 15 mins followed by PMA /ionomycin stimulation and measured for 1 hr by amplex red dye based fluorescence analysis
- ChEBML_152964 Inhibition of PMA and [Ca2+] induced 32P incorporation into histones by partially purified rat brain Protein kinase C
- ChEBML_162245 Inhibition of PMA and [Ca2+] induced 32P incorporation into histones by partially purified rat brain Protein kinase C
- ChEMBL_213813 (CHEMBL818858) In vitro inhibitory potency compared to PMA induced VCAM-1 expression in human endothelial cells (ELISA assay)
- ChEMBL_1469305 (CHEMBL3414202) Inhibition of PAD4 in C57BL/6 mouse bone marrow neutrophils assessed as inhibition of PMA-stimulated neutrophil extracellular trap formation preincubated for 30 mins followed by PMA stimulation measured after 3 to 4 hrs by DNA/neutrophil elastase overlap assay
- ChEMBL_515381 (CHEMBL1025536) Antagonist activity at TRPV1 expressed in HEK293 cells assessed as inhibition of PMA-induced activation by FLIPR assay
- ChEMBL_89061 (CHEMBL698941) In vitro inhibitory potency compared to PMA induced Intercellular adhesion molecule-1 in human endothelial cells (ELISA assay)
- ChEBML_102119 In vitro inhibitory activity against matrix metalloprotease 1 isolated from the culture medium of human skin fibroblasts induced with PMA
- ChEMBL_2470358 Agonist activity at TLR2 in PMA-differentiated human THP-1 cells assessed as increase in TNF-alpha level by ELISA
- ChEMBL_510614 (CHEMBL1002261) Inhibition of glucocorticoid-mediated human MMP1 expression in PMA stimulated A549 cells assessed as MMP1 protein level by ELISA
- ChEMBL_89060 (CHEMBL698940) In vitro inhibitory potency compared to PMA induced Intercellular adhesion molecule-1 expression in human endothelial cells (ELISA assay)
- ChEMBL_1863314 (CHEMBL4364170) Inhibition of MALT1-mediated T cell activation in human Jurkat cells assessed in decrease in PMA/ionomycin-induced IL2 production
- ChEMBL_2354137 Agonist activity at STING in PMA-differentiated human THP1-Dual KO-STING cells incubated for 24 hrs by QUANTI-Blue assay
- ChEMBL_2478779 Inhibition of NLRP3 in PMA-differentiated human THP-1 cells assessed as inhibition of LPS/nigericin induced IL-1beta production by ELISA
- ChEMBL_800279 (CHEMBL1948029) Inhibition of COX2 in LPS-stimulated and PMA-treated human U937 cells assessed as PGE2 production after 15 mins by ELISA
- ChEMBL_950755 (CHEMBL2353444) Inhibition of Myc signaling pathway in human HeLa cells assessed as inhibition of PMA induced luciferase treated 30 mins before induction
- ChEMBL_2310357 Agonist activity at human STING in human THP1-Blue ISG cells incubated for 24 hrs in presence of PMA by luciferase reporter assay
- ChEMBL_2310368 Agonist activity at mouse STING in mouse RAW-Lucia ISG cells incubated for 24 hrs in presence of PMA by luciferase reporter assay
- ChEMBL_2362809 Inhibition of NOX-2 dependent superoxide generation in PMA-differentiated human PLB-985 cells incubated for 1 hr by WST-1 based assay
- ChEMBL_2380211 Inhibition of NLRP3 in LPS/nigericin induced PMA-differentiated human THP-1 cells assessed as reduction in TNFalpha level measured after 3 hrs
- ChEMBL_499971 (CHEMBL1019341) Transrepression activity at GR in PMA-stimulated human A549 cells assessed as inhibition of AP1 response element-induced luciferase reporter gene activity
- ChEMBL_854680 (CHEMBL2161445) Inhibition of LCK in human Jurkat cells assessed as inhibition of PMA and antiCD3-induced IL2 secretion after 24 hrs by ELISA
- ChEMBL_983187 (CHEMBL2428099) Transrepression of glucocorticoid receptor in human A549 cells assessed as inhibition of PMA-induced AP-1 activity by luciferase reporter gene assay
- ChEBML_162244 In vitro inhibition of [32P] incorporation into histones by rat brain partially purified Protein kinase C in the presence of PMA, [Ca2+] and phosphatidylserine.
- ChEMBL_2343968 Agonist activity at STING in PMA-treated human THP-1 reporter cells incubated for 30 mins in presence of digitonin by QUANTI-Luc assay
- ChEMBL_2380186 Inhibition of NLRP3 in PMA-differentiated human THP-1 cells assessed as reduction in IL-1beta production measured after 18 hrs by HTRF assay
- ChEMBL_2380210 Inhibition of NLRP3 in LPS/nigericin induced PMA-differentiated human THP-1 cells assessed as reduction in IL-1beta production measured after 3 hrs
- ChEMBL_982006 (CHEMBL2428210) Agonist activity at glucocorticoid receptor in human A549 cells assessed as inhibition of PMA-induced AP-1 activation by luciferase reporter gene assay
- ChEMBL_983769 (CHEMBL2428108) Transrepression activity at glucocorticoid receptor in human A549 cells assessed as inhibition of PMA-stimulated AP1 response element by luciferase reporter gene assay
- ChEMBL_2310369 Agonist activity at human STING in STING knockout human THP1-Blue ISG cells incubated for 24 hrs in presence of PMA by luciferase reporter assay
- ChEMBL_2310370 Agonist activity at mouse STING in STING knockout mouse RAW-Lucia ISG cells incubated for 24 hrs in presence of PMA by luciferase reporter assay
- ChEMBL_2354127 Agonist activity at STING in PMA-differentiated wildtype human THP1-Blue ISG cells harboring IRF-inducible SEAP reporter construct incubated for 24 hrs by QUANTI-Blue assay
- ChEMBL_594764 (CHEMBL1039874) Transrepression activity at glucocorticoid receptor expressed in human A549 cells assessed as inhibition of PMA-induced AP1 activity after 6 hrs by luciferase reporter gene assay
- ChEMBL_754808 (CHEMBL1805663) Transrepression activity of glucocorticoid receptor in human A549 cells expressing AP-1 assessed as inhibition of PMA-induced AP-1 activity by luciferase reporter gene assay
- ChEMBL_2267144 Inhibition of mouse ADAM17 expressed in HEK293 cells overexpressed with ACE2 assessed as inhibition of ACE2 shedding incubated for 65 mins in presence of PMA by immunoblotting assay
- ChEMBL_2355736 Inhibition of human NOX1 transfected in CHO cells assessed as PMA-induced hydrogen peroxide pre-incubated for 10 and measured after 15 mins by Amplex-red based fluorescence assay
- ChEMBL_1760482 (CHEMBL4195490) Inhibition of PKC in TLR null HEK-blue cells assessed as inhibition of PMA-induced NF-kappaB activation-mediated SEAP production after 24 hrs by Quanti-blue-based assay
- ChEMBL_2355737 Inhibition of human NOX2 transfected in PLB-985 cells assessed as PMA-induced hydrogen peroxide pre-incubated for 10 and measured after 15 mins by Amplex-red based fluorescence assay
- ChEMBL_2355741 Inhibition of human DUOX1 transfected in HEK cells assessed as PMA-induced hydrogen peroxide production preincubated for 15 mins and measured after 10 mins by Amplex-red based fluorescence assay
- ChEMBL_2355742 Inhibition of human DUOX2 transfected in HEK cells assessed as PMA-induced hydrogen peroxide production preincubated for 15 mins and measured after 10 mins by Amplex-red based fluorescence assay
- ChEMBL_1759560 (CHEMBL4194568) Transrepression activity at glucocorticoid receptor (unknown origin) expressed in human ChaGoK1 cells assessed as inhibition of PMA-stimulated gene expression incubated for 24 hrs by beta-galactosidase reporter gene assay
- ChEMBL_1760483 (CHEMBL4195491) Inhibition of PKC in HEK-blue cells expressing human TLR7 assessed as inhibition of PMA-induced NF-kappaB activation-mediated SEAP production after 24 hrs by Quanti-blue-based assay
- ChEMBL_2355740 Inhibition of human NOX5 transfected in HEK cells assessed as PMA-induced hydrogen peroxide production pre-incubated for 10 mins and measured after 15 mins by Amplex-res based fluorescence assay
- ChEMBL_1824705 (CHEMBL4324469) Inhibition of human full-length eGFP-tagged RORgammat expressed in human HUT78 T-cells assessed as inhibition of PMA/CD3 monoclonal antibody stimulated IL-17 secretion after 48 hrs by ELISA
- ChEMBL_2278366 Modulation of glucocorticoid receptor (unknown origin) transfected in human HeLa cells assessed as transrepression by measuring inhibition of PMA-induced AP-1 signalling incubated for 18 hrs by dual-luciferase reporter based assay
- ChEMBL_2355738 Inhibition of human NOX3 transfected in HEK293-T-REx cells assessed as PMA-induced hydrogen peroxide production pre-incubated for 10 mins and measured after 15 mins by Amplex-res based fluorescence assay
- ChEMBL_2355739 Inhibition of human NOX4 transfected in HEK293-T-REx cells assessed as PMA-induced hydrogen peroxide production pre-incubated for 10 mins and measured after 15 mins by Amplex-res based fluorescence assay
- ChEMBL_1577623 (CHEMBL3807231) Inhibition of MALT1 (unknown origin) expressed in human Jurkat Clone K22 29Q_H23 cells assessed as IL2 production preincubated for 30 mins followed by PMA stimulation for 5.5 hrs by luciferase reporter gene assay
- ChEMBL_1680907 (CHEMBL4031184) Transrepression of glucocorticoid receptor in PMA-stimulated human ChaGoK1 cells expressing TRE-LacZ construct assessed as inhibition of AP-1 mediated TRE-LacZ activity after 24 hrs by beta-galactosidase reporter gene assay
- ChEMBL_1722939 (CHEMBL4137939) Inverse agonist activity at human full-length eGFP-tagged RORgammat expressed in human HUT78 T-cells assessed as inhibition of PMA/CD3 monoclonal antibody stimulated IL-17 secretion after 48 hrs by ELISA
- ChEMBL_1824393 (CHEMBL4324157) Inhibition of PRDX1 in human PMA-differentiated LPS-stimulated THP1 cells assessed as reduction in NLRP3 inflammasome activation preincubated for 1 hr followed by addition of ATP and mesured after 1 hr by ELISA
- ChEMBL_2299660 Inhibition of NLRP3 inflammasome activation in PMA-differentiated human THP-1 cells assessed as reduction in IL-1beta secretion preincubated for 20 hrs followed by nigericin addition and measured after 3 hrs by HTRF assay
- ChEMBL_2458308 Inhibition of NLRP3 in PMA-differentiated human THP-1 cells assessed as reduction in IL-1beta release preincubated with compound for 1 hrs followed by nigericin addition and measured after 3 hrs by HTRF assay
- ChEMBL_2475934 Inhibition of NLRP3 activation in PMA-differentiated LPS-stimulated human THP-1 cells assessed as reduction in IL-1beta release preincubated for 30 mins followed by nigericin addition and measured after 60 mins by ELISA
- ChEMBL_935203 (CHEMBL2317572) Inhibition of LFA-1/ICAM-1 interaction in mouse Lim51b cells assessed as PMA-induced adhesion to ICAM-1 expressing HSE cells measured per 10'5 cells after 30 mins by fluorometric microplate reader analysis
- ChEBML_1625692 Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduction in PMA-stimulated TRE-LacZ activity after 24 hrs in presence of MUG by fluorometric assay
- ChEMBL_2348002 Inhibition of NLRC4 (unknown origin) in PMA-differentiated human THP-1 cells assessed as inhibition of IL-1 beta production pretreated for 15 mins followed by flagellin stimulation and measured after 18 hrs by AlphaLISA assay
- ChEMBL_1625692 (CHEMBL3868161) Agonist activity at glucocorticoid receptor in human ChaGoK1 cells assessed as inhibition of AP1-mediated transcriptional activity by measuring reduction in PMA-stimulated TRE-LacZ activity after 24 hrs in presence of MUG by fluorometric assay
- ChEMBL_2128481 (CHEMBL4837910) Inhibition of ASK1 in human HEK293 cells transfected with AP1 assessed as inhibition of AP1-induced firefly luciferase activity preincubated for 1 hr followed by PMA stimulation and measured after 6 hrs by luciferase reporter assay
- ChEMBL_2358694 Inhibition of NLRP3 inflammasome in PMA-differentiated human THP-1 cells assessed as inhibition of Pam3CSK4-induced TNF-alpha secretion pretreated with compound for 1 hr followed by stimulation with Pam3CSK4 for 3 hrs by HTRF assay
- ChEMBL_2358693 Inhibition of NLRP3 inflammasome in PMA-differentiated human THP-1 cells assessed as inhibition of nigericin-induced IL-1 beta secretion pretreated with compound for 1 hr followed by stimulation with nigericin for 3 hrs by HTRF assay
- ChEMBL_1824400 (CHEMBL4324164) Inhibition of NLRP3 inflammasome activation in PMA differentiated human THP1 cells assessed as reduction in IL-1beta level preincubated for 30 mins followed by addition of MSU and meaured after 6 hrs by ELISA method relative to control
- ChEMBL_2094023 (CHEMBL4775286) Inhibition of NLRP3 in PMA-stimulated human THP-1 cells assessed as reduction in IL-1beta release pre-treated for 24 hrs followed by stimulation with LPS for 3 hrs and ATP for 1 hr by ELISA analysis
- ChEMBL_2209700 (CHEMBL5122649) Inhibition of NLRP3 inflammasome activation in PMA differentiated LPS-primed human THP-1 cells assessed as reduction in nigericin-induced IL-1beta level pretreated for 2 hrs followed by nigericin addition and measured after 30 mins by ELISA
- ChEMBL_2048179 (CHEMBL4702878) Inhibition of NLRP3 inflammasome activation in LPS-primed human PMA-differentiated THP-1 cells assessed as reduction in nigericin-induced IL-1beta level preincubated for 30 mins followed by nigericin addition and measured after 1 hrs by Western blot analysis
- ChEMBL_1715620 (CHEMBL4125669) Inhibition of nigericin-induced NLRP3 inflammasome activation in PMA-differentiated human THP1 cells assessed as reduction in LPS-stimulated IL-1beta secretion incubated for 1 hr prior to stimulation with nigericin for 1 hr by quanti-blue based SEAP reporter gene assay
- ChEMBL_2224861 (CHEMBL5138374) Inhibition of NLRP3 inflammasome activation in LPS/nigericin-stimulated PMA-differentiated human THP-1 macrophages assessed as reduction in pyroptosis pretreated with LPS followed by compound treatment for 30 mins then stimulation with nigericin and measured after 3 hrs by MTT assay
- ChEMBL_2326165 Inhibition of NLRP3-dependent pyroptosis in PMA-differentiated human THP-1 cells assessed as inhibition of LPS/ATP-induced cell death pretreated for 4 hrs with LPS followed by incubation with compound for 1 hr and later treated with ATP for 1.5 hrs by LDH assay
- ChEMBL_2358629 Inhibition of NLRP3 inflammasome in PMA-differentiated human THP-1 cells assessed as inhibition of LPS/nigericin-induced IL-1 beta secretion pretreated with LPS for 3 hrs followed by incubation with compound for 40 mins and later stimulated with nigericin for 35 mins by ELISA
- ChEMBL_2478784 Inhibition of NLRP3 inflammasome activation in PMA-differentiated human THP-1 cells assessed as inhibition of LPS/nigericin induced IL-1beta production preincubated with LPS for 4 hrs followed by compound addition for 30 mins and further incubated with nigericin for 30 mins by ELISA
- ChEMBL_2326166 Inhibition of NLRP3 inflammasome activation in PMA-differentiated human THP-1 cells assessed as inhibition of LPS/ATP-induced IL-1 beta release pretreated for 4 hrs with LPS followed by incubation with compound for 1 hr and later treated with ATP for 1.5 hrs by ELISA
- ChEMBL_2438144 Inhibition of NLRP3 inflammasome activation in LPS primed human differentiated THP-M cells derived PMA induced THP-1 cells assessed as inhibition of IL-1 beta level preincubated with compound for 24 hrs followed by LPS stimulation for 3 hrs and further incubated with ATP for 1 hr by ELISA method
- Cell-Based Enzyme Inhibition Assay (ELISA) Detection of the effect of compounds on p-MEK1/2, t-ERK1/2, pERK1/2, p-Rsk1 and p-Elk-1 activity in B16-F10 cells was performed using ELISA kits (Invitrogen)and strictly according to the manufacturer's instructions. Cells were pre-treated with 30 nM PMA (Sigma) in the absence or presence of these compounds.
- IL-2 ELISA Assay Primary T cell stimulation and IL2 ELISA: Human primary T cells (100,000 cells per well) were pre-incubated with or without test compound in Yssel's medium for 1 hour at 37° C. Cells were then stimulated by transferring them to round-bottom 96-well plates pre-coated with 1 μm/ml αCD3 and 5 μg/ml αCD28. For counter assay, cells were instead stimulated by adding 8× stock solutions of PMA and ionomycin in Yssels (for final concentrations of 0.5 ng/ml PMA and 0.1 uM ionomycin, both from Calbiochem). Cells were incubated at 37° C. for 24 hours before 100 μL supernatants were harvested for quantification of IL-2 by ELISA using Human IL-2 Duoset ELISA Kit from R and D Systems, Cat. # DY202E.
- Protein Kinase C beta 2 Assay A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of assay buffer containing 50 mM HEPES, pH 7.4, 5 nM PKC, 23 units of pyruvate kinase, 33 units of lactate dehydrogenase, 0.15 mM peptide, 0.1 mM ATP, 1 mM DTT, 4 mM PEP, 8 mM MgCl2, 0.3 mM NADH, 60 mM CaCl2, 10 mg/mL PS, 50 ng/mL PMA, 7.5% DMSO and from about 10,000 nM to 0.169 nM compound inhibitor. Stock solutions of 3-sn-phosphatidyl-L-serine (PS) and phorbol-12-myristate-13-acetate (PMA) were sonicated for 30 seconds just prior to addition to assay buffer and assays were initiated by the addition of 100 μM ATP.
- In vivo Deuterated 1-Butanol PLD Assay Cells were seeded into 12-well tissue culture plates to reach 90% confluence at the time of assay. All cell types, aside from the HEK293-gfpPLD2 cells, were serum-starved 18 h prior to experiment in DMEM, 0.5% FBS, 1% AA. Cells werepretreated in the presence of test compound (50 μM to 5 nM) or DMSO (vehicle control) in DMEM for 5 min at rt. After pretreatment, media was removed, and cells were treated with DMEM + 1 μM PMA + 0.3% 1-butanol-d10, and either test compound, DMSO vehicle control, or DMEM alone for 30 min at 37 °C. HEK293-gfpPLD2 cells were treated in the presence of DMEM + 0.3% 1-butanol-d10 and either test compound or DMSO but were not stimulated with PMA. After treatment, samples were extracted, and 1,2-dihexanoyl-snglycero-3-phosphomethanol (32:0 PtdMeOH) was added as an internal standard. The lipids were isolated, the solvent was evaporated, and the resulting lipid film was resuspended in MS solvent (9:1 methanol/chloroform + 1 μL of NH4OH). Samples were
- Phosphorylation Assay HepG2 cells were obtained from ATCC and grown in DMEM supplemented with 10% fetal bovine serum. Cells were plated in 96-well plates at 35,000 cells/well and allowed to attach overnight at 37° C./5% CO2. Diluted compounds were then added at a final concentration of 0.5% DMSO. After 1.5 hour compound incubation, cells were stimulated with the addition of PMA (phorbol 12-myristate 13-acetate) at a final concentration of 100 ng/mL; the PMA stimulation was a 30-minute incubation at 37° C./5% CO2. After the 30-minute PMA stimulation, cells were washed with PBS and fixed in 3.7% formaldehyde in PBS at room temperature for 15-20 minutes. This was followed by another wash in PBS and then permeabilization in 100% MeOH at room temperature for 10-15 minutes. Following the permeabilization incubation, cells were washed in PBS/0.05% Tween-20, followed by a block in Odyssey blocking buffer (LI-COR Biosciences) for at least 1 hour. Antibodies to phosphorylated P90RSK(Ser380) (Cell Signaling #9335, rabbit monoclonal) and GAPDH (Fitzgerald 10R-G109a, mouse monoclonal) were added to the cells and incubated overnight at 4° C. pP90RSK(Ser380) antibody was used at a 1:250 dilution; GAPDH was used at a 1:10,000 dilution. After washing with PBS/0.05% Tween-20, the cells were incubated with fluorescently-labeled secondary antibodies (Anti-rabbit-Alexa Flour680, Invitrogen Cat#A21109; Anti-mouse-IRDye800CW, Rockland Inc. Cat#610-131-121) for 1 hour. Both secondary antibodies were used at a 1:1000 dilution. Cells were then washed and analyzed for fluorescence at both wavelengths using the Odyssey Infrared Imaging System (LI-COR Biosciences). Phosphorylated P90RSK(Ser380) signal was normalized to GAPDH signal.
- Protein Kinase C beta 2 (PKCpII) Assay Protein Kinase C beta 2 (PKCpII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate peptide (A→S, RFARKGSLRQKNV). This transfer is coupled to the oxidation of β-NADH through the activities of Pyruvate Kinase (PK) and Lactate Dehydrogenase (LDH). β-NADH conversion to NAD+ is monitored by the decrease in absorbance at 340 nm (e=6.22 cm−1 mM−1) using a Molecular Devices SPECTRA max PLUS spectrophotometer.A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of assay buffer containing 50 mM HEPES, pH 7.4, 5 nM PKC, 23 units of pyruvate kinase, 33 units of lactate dehydrogenase, 0.15 mM peptide, 0.1 mM ATP, 1 mM DTT, 4 mM PEP, 8 mM MgCl2, 0.3 mM NADH, 60 mM CaCl2), 10 mg/mL PS, 50 ng/mL PMA, 7.5% DMSO and from about 10,000 nM to 0.169 nM compound inhibitor. Stock solutions of 3-sn-phosphatidyl-L-serine (PS) and phorbol-12-myristate-13-acetate (PMA) were sonicated for 30 seconds just prior to addition to assay buffer and assays were initiated by the addition of 100 μM ATP.
- ROS Inhibiting Drug A high-throughput screen was performed to find molecules that inhibit ROS production by neutrophils. Extracted human neutrophils were purified and kept in culture. The cells were then exposed to various drugs and ROS production was monitored over time. Compounds that also scavenged hydrogen peroxide (H2O2) and/or lowered neutrophil ATP levels (reflecting toxicity) were removed. The top hits from the screen were selected for further analysis.160 basal hits were tested for their ability to inhibit neutrophil ROS production. 64 molecules were able to inhibit ROS production in the presence of PMA activation. 67 molecules were able to inhibit ROS production in the presence of N-Formylmethionine-leucyl-phenylalanine (fMLP). Of those 47 molecules were able to inhibit ROS production by both stimulation methods.
- In-Vitro Assay THP1 monocytes were differentiated with PMA (100 ng/mL) and incubated at 37 deg C. for 20 hrs in presence of 5% CO2. 2×105 differentiated cells were plated per well of 96 well tissue culture plates. The cells were primed using 500 ng/mL Lipopolysaccharide and incubating for 4 h under the same condition. The cells were then treated with various concentrations of the compounds for 30 min followed by treatment with 5 mM ATP for 1 hr. The supernatants were collected and analyzed by IL-1b (Mabtech Cat #3415-1H-20) or TNF-α (Mabtech; Cat #3510-1H-20) detection kit. The data were analyzed using GraphPad Prism V7.0. Dose Response Curve (DRC) was constructed to determine the IC50 value by fitting percentage cell survival data to the GraphPad Prism using nonlinear regression analysis.
- Nox2 Assay Cells: Human blood was purchased in buffy coat, prepared the same day for isolation of neutrophils, from Labjoy AB, Lund, Sweden. Blood components were separated by density gradient centrifugation using Ficoll-Paque Plus. Plasma, PBMCs and Ficoll were removed before erythrocytes were removed by dextran sedimentation. Remaining erythrocytes were lyses before neutrophils were washed and counted. Isolated neutrophils were kept on ice resuspended in HBSS without Mg and Ca until assayed.Buffers: The isoluminol buffer contained Isoluminol (0.175 mg/ml) and HRP fraction II (1.75 U/ml). The buffer was prepared by diluting these ingredients at 4× working concentration in HBSS.Procedures: Compounds (Nox inhibitors) were diluted at 4× working concentration and titrated from 100 μM to 0.006 μM in 1:4 steps. PMA was diluted in Isoluminol buffer at 4× working concentration for a final concentration of 30 ng/ml. Compounds had a final DMSO concentration of 1% in the wells; therefore a DMSO control of 1% was included on the plates. 25 μl diluted compound or control/well were added to a white 96-well plate. 25 μl/well of PMA diluted in Isoluminol buffer was added to each well. To non-stimulated control wells only Isoluminol buffer was added. Neutrophils were washed and resuspended at 2×106 cells/ml in HBSS with Mg and Ca just before adding 50 μl of the neutrophil cell suspension/well, which was followed by immediate initiation of luminescence measurement. Luminescence was measured using a FluoStar Optima (BMG Labtech). Graphs were performed using Prism 5 for Mac OS X (Prism 5.0 Software, San Diego Calif. USA). Inhibitors were evaluated at 50% inhibition (IC50) in comparison to cell control without inhibitor present.
- In-Vitro Assay THP1 (Tamm-Horsfall Protein 1) monocytes were differentiated with PMA (Phorbol 12-myristate 13-acetate) (100 ng/ml) and incubated at 37° C. for 20 h in presence of 5% CO2. 2×105 differentiated cells were plated per well of 96 well tissue culture plates. The cells were primed using 500 ng/ml Lipopolysaccharide and incubating for 4 hrs under the same condition. The cells were then treated with various concentrations of the compounds for 30 min followed by treatment with 5 mM ATP for 1 hr. The supernatants were collected and analysed by IL-1b (Mabtech Cat #3415-1H-20) or TNF-a (Mabtech; Cat #3510-1H-20) detection kit. The data were analyzed using GraphPad Prism V7.0. Dose Response Curve (DRC) was constructed to determine the IC50 value by fitting percentage cell survival data to the GraphPad Prism using nonlinear regression analysis.
- SAR analysis of NF-kappaB dependent luciferase using PMA/Ionomycin as an inducer - 2 Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Probe Production Centers Network (MLPCN) Grant Number: 1 R03 MH084844-01 Assay Provider: Dr. John C. Reed, Sanford-Burnham Medical Research Institute, San Diego CA The modulation of immune response activity is one of the major goals in the development of novel therapeutics for auto-immune and inflammatory diseases. The innate system resides at the intersection of the pathways of microbial recognition, inflammation, and cell death, thereby offering various therapeutic targets. In this context, NOD1 and NOD2 are of particular interest, since they recognize distinct structures derived from bacterial peptidoglycans and directly activate NF-kB, a central regulator of immune response, inflammation, and apoptosis. NF-kB pathway activated by antigen receptors is critical for acquired (as opposed to innate)
- Inhibition Assay Protein Kinase C beta 2 (PKCβII) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate peptide (A→S, RFARKGSLRQKNV). This transfer is coupled to the oxidation of β-NADH through the activities of Pyruvate Kinase (PK) and Lactate Dehydrogenase (LDH). β-NADH conversion to NAD+ is monitored by the decrease in absorbance at 340 nm (e=6.22 cm−1 mM−1) using a Molecular Devices SPECTRA max PLUS spectrophotometer.A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30° C. in 0.1 mL of assay buffer containing 50 mM HEPES, pH 7.4, 5 nM PKC, 23 units of pyruvate kinase, 33 units of lactate dehydrogenase, 0.15 mM peptide, 0.1 mM ATP, 1 mM DTT, 4 mM PEP, 8 mM MgCl2, 0.3 mM NADH, 60 mM CaCl2, 10 mg/mL PS, 50 ng/mL PMA, 7.5% DMSO and from about 10,000 nM to 0.169 nM compound inhibitor. Stock solutions of 3-sn-phosphatidyl-L-serine (PS) and phorbol-12-myristate-13-acetate (PMA) were sonicated for 30 seconds just prior to addition to assay buffer and assays were initiated by the addition of 100 μM ATP.Steady-state kinetic parameters for the bi-bi kinase reaction were determined at saturating phospho-acceptor peptide substrate concentration (0.15 mM) by fitting initial velocity data to the Michaelis-Menten equation, v=V max [S]/(K M +[S]) where v is the measured initial velocity, Vmax is the maximal enzyme velocity, [S] is the ATP substrate concentration, and KM is the Michealis constant for ATP. Enzyme turnover values (kcat) were calculated according to kcat=Vmax[E], where [E] is the total enzyme concentration. Enzyme inhibition constants (apparent Ki values) were determined by fitting initial velocities at variable inhibitor concentrations to a model for ATP competitive inhibition based on the Morrison equation). Morrison, J. F., Biochim. Biophys Acta 185: 269-286 (1969).
- hTHP-1 assay THP-1 cells were purchased from the American Type Culture Collection and sub-cultured according to instructions from the supplier. Prior to experiments, cells were cultured in complete RPMI 1640 (containing 10% heat inactivated FBS, penicillin (100 units/ml) and streptomycin (100 μg/ml)), and maintained in log phase prior to experimental setup. Prior to the experiment THP-1 were treated with PMA (Phorbol 12-myristate 13-acetate) (20 ng/ml) for 16-18 hours. Compounds were dissolved in dimethyl sulfoxide (DMSO) to generate a 30 mM stock. On the day of the experiment the media was removed and adherent cells were detached with trypsin for 5 minutes. Cells were then harvested, washed with complete RPMI 1640, spun down, resuspended in RPMI 1640 (containing 2% heat inactivated FBS, penicillin (100 units/ml) and streptomycin (100 μg/ml). The cells were plated in a 384-well plate at a density of 50,000 cells/well (final assay volume 50 μl). Compounds were first dissolved in assay medium to obtain a 5× top concentration of 500 μM. 10 step dilutions (1:3) were then undertaken in assay medium containing 1.67% DMSO. 5× compound solutions were added to the culture medium to achieve desired final concentration (e.g. 100, 33, 11, 3.7, 1.2, 0.41, 0.14, 0.046, 0.015, 0.0051, 0.0017 M). Final DMSO concentration was at 0.37%. Cells were incubated with compounds for 1 hour and then stimulated with gramicidin (5 μM) (Enzo) for 2 hours. Plates were then centrifuged at 340 g for 5 min. Cell free supernatant (40 μL) was collected using a 96-channel PlateMaster (Gilson) and the production of IL-1β was evaluated by HTRF (cisbio). A vehicle only control and a dose titration of CRID3 (100-0.0017 μM) were run concurrently with each experiment. Data was normalized to vehicle-treated samples (equivalent to 0% inhibition) and CRID3 at 100 μM (equivalent to 100% inhibition). Compounds exhibited a concentration-dependent inhibition of IL-1β production in PMA-differentiated THP-1 cells.
- Cell Based Assay As a means of assessing PI3K δ activation in response to stimuli, the phosphorylation status of the protein, Akt, a downstream product of PI3Kδ, signaling was determined.U937 cells, obtained from a human, leukemic, monocyte lymphoma cell line, were differentiated to macrophage-type cells by incubation with PMA (100 ng/mL) for 48 to 72 hr. Cells were then pre-incubated with either the test compound or vehicle for 2 hr and were then stimulated briefly by exposure to H2O2 (10 mM, 5-7 min) and the reaction stopped by replacing the media with 4% formaldehyde solution. Endogenous peroxide activity and formaldehyde were inactivated by incubating with quenching buffer (0.1% sodium azide, 1% H2O2 in PBS with 0.1% Triton X-100) for 20 min. The cells were washed with buffer (PBS containing 0.1% Triton X-100) and were incubated with blocking solution (1% BSA in PBS) for 1 hr and were then re-washed with buffer and incubated overnight with either anti-pAkt antibody or anti-pan-Akt antibody.
- Cell Based Assay As a means of assessing the activation of PI3K γ in response to stimuli, the phosphorylation status of the protein, Akt, a downstream product of PI3K γ signalling, was determined following stimulation with MCP-1.U937 cells were differentiated to macrophage-type cells by incubation with PMA (100 ng/mL) for 48 to 72 hr. Cells were then pre-incubated with either the test compound or vehicle for 2 hr and were then stimulated briefly with MCP-1 (10 nM, 1 min) and the reaction stopped by replacing the media with 4% formaldehyde solution. Endogenous peroxide activity and formaldehyde were inactivated by incubating with quenching buffer (0.1% sodium azide, 1% H2O2 in PBS with 0.1% Triton X-100) for 20 min. The cells were washed with buffer (PBS containing 0.1% Triton X-100) and were incubated with blocking solution (1% BSA in PBS) for 1 hr and were then re-washed with buffer and incubated overnight with either anti-pAkt antibody or anti-pan-Akt antibody.
- IL-1 Secretion Assa Monocytic THP-1 cells (ATCC: TIB-202) were maintained according to providers' instructions in RPMI media (RPMI/Hepes+10% fetal bovine serum+Sodium Pyruvate+0.05 mM Beta-mercaptoethanol (1000× stock)+Pen-Strep). Cells were differentiated in bulk with 0.5 μM phorbol 12-myristate 13-acetate (PMA; Sigma #P8139) for 3 hours, media was exchanged, and cells were plated at 50,000 cells per well in a 384-well flat-bottom cell culture plates (Greiner, #781986), and allowed to differentiate overnight. Compound in a 1:3.16 serial dilution series in DMSO was added 1:100 to the cells and incubated for 1 hour. The NLRP3 inflammasome was activated with the addition of 15 μM (final concentration) Nigericin (Enzo Life Sciences, #BML-CA421-0005), and cells were incubated for 3 hours. 10 μL supernatant was removed, and IL-1p levels were monitored using an HTRF assay (CisBio, #621L1PEC) according to manufacturers' instructions. Viability and pyroptosis was monitored with the addition of PrestoBlue cell viability reagent (Life Technologies, #A13261) directly to the cell culture plate.
- Inhibition Assay Protein Kinase C beta 2 (PKC beta II) catalyzes the production of ADP from ATP that accompanies the phosphoryl transfer to the PKC Pseudosubstrate peptide (A->S, RFARKGSLRQKNV). This transfer is coupled to the oxidation of p-NADH through the activities of Pyruvate Kinase (PK) and Lactate Dehydrogenase (LDH). (3-NADH conversion to NAD+ is monitored by the decrease in absorbance at 340 nm (e=6.22 cm-1 mM-1) using a Molecular Devices SPECTRA max PLUS spectrophotometer.A typical assay was carried out on a 96-well, clear microtiter plate in a Molecular Devices spectrophotometer for 20 minutes at 30 C. in 0.1 mL of assay buffer containing 50 mM HEPES, pH 7.4, 5 nM PKC, 23 units of pyruvate kinase, 33 units of lactate dehydrogenase, 0.15 mM peptide, 0.1 mM ATP, 1 mM DTT, 4 mM PEP, 8 mM MgCl2, 0.3 mM NADH, 60 mM CaCl2, 10 mg/mL PS, 50 ng/mL PMA, 7.5% DMSO and from about 10,000 nM to 0.169 nM compound inhibitor.
- Nox1 Assay CHO cells modified to stably express human Nox1 were grown in DMEM/F12 gibco 31331 containing 10% FBS and 1% pen/strep at 37° C. in air with 5% CO2. Cells were collected from cultures by Trypsin mediated detachment of adherent cells.A luminescence assay was used that measures the production of reactive oxygen species in whole cells. Luminol reacts with superoxide and emits light and light is measured with luminometer (Synergy/2 microplate reader, BioTek).Inhibitors were diluted in a compound plate in DMSO (100%) then transferred to Hanks buffer solution and in assay plate DMSO were 2% in all the wells.Assay procedure, final well volume 100 μl, 96-well plate: Inhibitors (20 μl) were added, then cell suspension was (100 000 cells/well), incubate 37° C. for 30 min, add PMA (0.9 μM/well) to Luminol reaction mix (Luminol 0.1 mM/well and HRP 3.2 U/well) then this stimulation mix into wells. The plate were then immediately read (steps 5 min each reading) and for 1 h. Data was calculated for the linear part of the curve and IC50 determined.Compounds (Nox inhibitors) were diluted at 3× working concentration and titrated from 200 μM to 0.003 μM in 11 steps.
- IL-1beta Release Assay The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to active IL-1β. The functional activity of P2X7 compounds was measured by the release of mature IL-1β in the culture medium of THP-1 cells, detected by sandwich ELISA. Cells were maintained in complete growth medium (RPMI 1640+10% HI-FCS+2 mM L-glutamine+1×PS). Every 3 days, the medium was renewed by diluting the cells 1/3 to 1/4 as cell density did not exceed 0.5 million cells per ml (seeding cell density @ 1×105/ml). THP-1 cells were harvested from the flask in 50 ml by centrifugation for 3 min at 100 g. The cells were resuspended to 2×105 cells/ml in medium supplemented with 0.5 μM PMA and incubated. The cells were washed and resuspended to 1.5×105 cells/ml in medium complemented with 10 ng/ml LPS, and the cells were primed for 4 h at 37° C., 5% CO2. After addition of 20 μL of prediluted test compounds, blank, standard and control reagents, cells were incubated for a further 20 min at 37° C. and stimulated with 0.8 mM BzATP for 30 minutes. The cells were centrifuged, supernatant was collected and the presence of mature IL-1β was detected using Dual human IL-1b kit following manufacturer's instruction. The tetrahydrobenzodiazepine analogs effectively modulated the activity of P2X7 in the cells as measured by the levels of pro-inflammatory cytokine IL-1β, which is released by the activation of P2X7 receptor.
- IL-1beta release assay The activation of P2X7 by ATP leads to a fast transient activation of cells resulting in influx of Ca2+ followed by conversion of pro-IL-1β to active IL-1β. The functional activity of P2X7 compounds was measured by the release of mature IL-13 in the culture medium of THP-1 cells, detected by sandwich ELISA. Cells were maintained in complete growth medium (RPMI 1640+10% HI-FCS+2 mM L-glutamine+1×PS). Every 3 days, the medium was renewed by diluting the cells 1/3 to 1/4 as cell density did not exceed 0.5 million cells per ml (seeding cell density @1×105/ml). THP-1 cells were harvested from the flask in 50 ml by centrifugation for 3 min at 100 g. The cells were resuspended to 2×105 cells/ml in medium supplemented with 0.5 μM PMA and incubated. The cells were washed and resuspended to 1.5×105 cells/ml in medium complemented with 10 ng/ml LPS, and the cells were primed for 4 h at 37° C., 5% CO2. After addition of 20 μL of prediluted test compounds, blank, standard and control reagents, cells were incubated for a further 20 min at 37° C. and stimulated with 0.8 mM BzATP for 30 minutes. The cells were centrifuged, supernatant was collected and the presence of mature IL-1β was detected using Dual human IL-1b kit following manufacturer's instruction. The tetrahydrobenzodiazepine analogs effectively modulated the activity of P2X7 in the cells as measured by the levels of pro-inflammatory cytokine IL-1β, which is released by the activation of P2X7 receptor.
- Evaluation of NLRP3 Inflammasome Inhibitory Activity Assay The NLRP3 inflammasome inhibitory activity of test compounds were evaluated CM the basis of the inhibitory activity of the IL-1β, production in THP1-Null cells (Product Number: thp-null, InvivoGen). Cells were maintained for culture in RPMI-1640 media containing 10% (v/v) fetal bovine serum, 25 mmol/1. RUES, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μg/ml normocin, and 200 μg/mL hygromycin B (set at 37° C., 5% CO2/95% air). Cells were suspended with media for assay containing 0.5 μmol/L PMA (RPMI-1640 media containing 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin), and the suspended cells were seeded on Corning (registered trademark) 384-well Flat. Clear Bottom Black Polystyrene TC-treated Microplates (25,000 cells/25 μL/well)(followed by incubation (set at 37° C., 5% CO2/95% air) overnight supernatant of the culture was removed, and thereto was added media for assay (25 UL/well) containing 1 μg/mL Lipopolysaccharides (Product Number: L2654, Sigma-Aldrich (registered trademark)). Then, the culture was further incubated for 3 hours (set at 37° C., 5% CO2/91% air). The supernatant of the culture was removed. Then, a vehicle solution prepared from Opti-MEM (trademark) medium (Product Number: 31985-070, Invitrogen) was added to blank-setting wells and control-setting wells (20 μL/well), followed by incubation for 15 minutes (set at 37° C., 5% CO2/91% air). A solution containing a test compound (20 μL/well) was added to test compound-setting wells. Further, Opti-MEM (trademark) medium containing Nigericin (Product Number: N7143, Sigma-Aldrich (registered trademark)) was added to the control-setting wells and test compound-setting wells (5 μL/well), followed by incubation for 1.5 hours (set at 37° C., 5% CO2/95% air). The final concentration of Nigericin was adjusted to be 7.5 μmol/L, 5 μL/well of Opti-MEM (trademark) medium was added to the blank setting wells. The supernatant of the culture was cryonically stored (set at −20° C.) until measurement of IL-1β.
- Evaluation of NLRP3 Inflammasome Inhibitory Activity Assay The NLRP3 inflammasome inhibitory activity of test compounds were evaluated on the basis of the inhibitory activity of the IL-1β production in THP1-Null cells (Product Number: thp-null, InvivoGen). Cells were maintained for culture in RPMI-1640 media containing 10% (v/v) fetal bovine serum, 25 mmol/L HEPES, 100 U/mL penicillin, 100 μg/mL streptomycin, 100 μg/mL normocin, and 200 μg/mL hygromycin B (set at 37° C., 5% CO2/95% air). Cells were suspended with media for assay containing 0.5 μmol/L PMA (RPMI-1640 media containing 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin), and the suspended cells were seeded on Corning (registered trademark) 384-well Flat Clear Bottom Black Polystyrene TC-treated Microplates (25,000 cells/25 μL/well), followed by incubation (set at 37° C., 5% CO2/95% air) overnight. The supernatant of the culture was removed, and thereto was added media for assay (25 μL/well) containing 1 μg/mL Lipopolysaccharides (Product Number: L2654, Sigma-Aldrich (registered trademark)). Then, the culture was further incubated for 3 hours (set at 37° C., 5% CO2/95% air). The supernatant of the culture was removed. Then, a vehicle solution prepared from Opti-MEM (trademark) medium (Product Number: 31985-070, Invitrogen) was added to blank-setting wells and control-setting wells (20 μL/well), followed by incubation for 15 minutes (set at 37° C., 5% CO2/95% air). A solution containing a test compound (20 μL/well) was added to test compound-setting wells. Further, Opti-MEM (trademark) medium containing Nigericin (Product Number: N7143, Sigma-Aldrich (registered trademark)) was added to the control-setting wells and test compound-setting wells (5 μL/well), followed by incubation for 1.5 hours (set at 37° C., 5% CO2/95% air). The final concentration of Nigericin was adjusted to be 7.5 μmol/L. 5 μL/well of Opti-MEM (trademark) medium was added to the blank-setting wells. The supernatant of the culture was cryonically stored (set at −20° C.) until measurement of IL-1β.
 BDBM512625 PMA
BDBM512625 PMA para-Methoxyamphetamine (S)-(+)2-(4-Methoxy-phenyl)-1-methyl-ethylamine (-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine(PMA) (R)-(-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine BDBM50024209 (+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine CHEMBL278663 beta-methoxyamphetamine
para-Methoxyamphetamine (S)-(+)2-(4-Methoxy-phenyl)-1-methyl-ethylamine (-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine(PMA) (R)-(-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine BDBM50024209 (+/-)2-(4-Methoxy-phenyl)-1-methyl-ethylamine 2-(4-Methoxy-phenyl)-1-methyl-ethylamine CHEMBL278663 beta-methoxyamphetamine