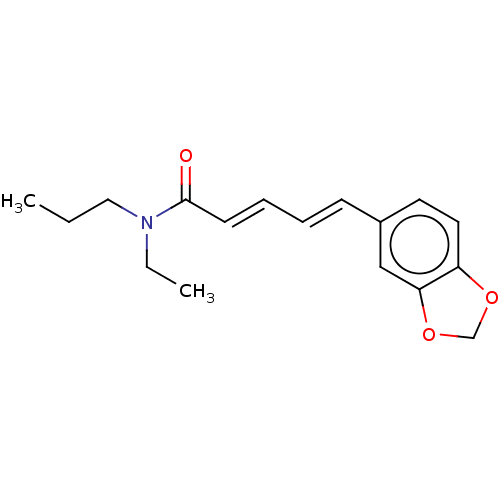
 US9144538, Piperine US9138393, Piperine BDBM181116
US9144538, Piperine US9138393, Piperine BDBM181116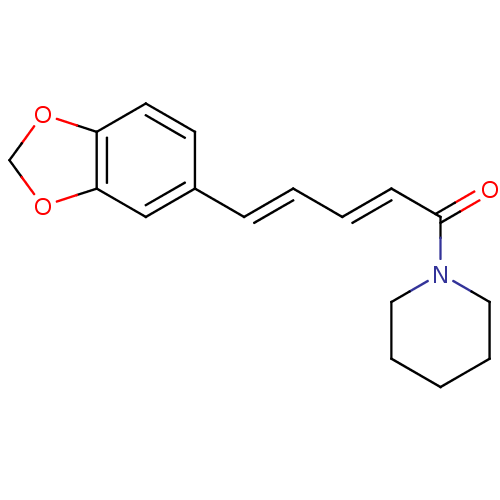 N-[(E,E)-piperoyl]piperidine 1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)penta-2,4-dienoyl]piperidine CHEMBL43185 piperine BDBM50148573 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-2,4-pentadienoyl]piperidine (E,E)-1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine
N-[(E,E)-piperoyl]piperidine 1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)penta-2,4-dienoyl]piperidine CHEMBL43185 piperine BDBM50148573 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-2,4-pentadienoyl]piperidine (E,E)-1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine
- Correa, EA; Högestätt, ED; Sterner, O; Echeverri, F; Zygmunt, PM In vitro TRPV1 activity of piperine derived amides. Bioorg Med Chem 18: 3299-306 (2010)
- Mu, LH; Wang, B; Ren, HY; Liu, P; Guo, DH; Wang, FM; Bai, L; Guo, YS Synthesis and inhibitory effect of piperine derivates on monoamine oxidase. Bioorg Med Chem Lett 22: 3343-8 (2012)
- Sethi, KK; Sahoo, SK; Pichikala, JN; Suresh, P Carbonic anhydrase I and II inhibition with natural products: caffeine and piperine. J Enzyme Inhib Med Chem 27: 97-100 (2012)
- Schöffmann, A; Wimmer, L; Goldmann, D; Khom, S; Hintersteiner, J; Baburin, I; Schwarz, T; Hintersteininger, M; Pakfeifer, P; Oufir, M; Hamburger, M; Erker, T; Ecker, GF; Mihovilovic, MD; Hering, S Efficient modulation of γ-aminobutyric acid type A receptors by piperine derivatives. J Med Chem 57: 5602-19 (2014)
- Tomy, MJ; Sharanya, CS; Dileep, KV; Prasanth, S; Sabu, A; Sadasivan, C; Haridas, M Derivatives form better lipoxygenase inhibitors than piperine: in vitro and in silico study. Chem Biol Drug Des 85: 715-21 (2015)
- Bhardwaj, RK; Glaeser, H; Becquemont, L; Klotz, U; Gupta, SK; Fromm, MF Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther 302: 645-50 (2002)
- Rahman, T; Rahmatullah, M Proposed structural basis of interaction of piperine and related compounds with monoamine oxidases. Bioorg Med Chem Lett 20: 537-40 (2010)
- Elimam, DM; Elgazar, AA; Bonardi, A; Abdelfadil, M; Nocentini, A; El-Domany, RA; Abdel-Aziz, HA; Badria, FA; Supuran, CT; Eldehna, WM Natural inspired piperine-based sulfonamides and carboxylic acids as carbonic anhydrase inhibitors: Design, synthesis and biological evaluation. Eur J Med Chem 225: (2021)
- Zhu, P; Qian, J; Xu, Z; Meng, C; Liu, J; Shan, W; Zhu, W; Wang, Y; Yang, Y; Zhang, W; Zhang, Y; Ling, Y Piperlonguminine and Piperine Analogues as TrxR Inhibitors that Promote ROS and Autophagy and Regulate p38 and Akt/mTOR Signaling. J Nat Prod 83: 3041-3049 (2020)
- Chavarria, D; Fernandes, C; Silva, V; Silva, C; Gil-Martins, E; Soares, P; Silva, T; Silva, R; Remião, F; Oliveira, PJ; Borges, F Design of novel monoamine oxidase-B inhibitors based on piperine scaffold: Structure-activity-toxicity, drug-likeness and efflux transport studies. Eur J Med Chem 185: (2020)
- Al-Baghdadi, OB; Prater, NI; Van der Schyf, CJ; Geldenhuys, WJ Inhibition of monoamine oxidase by derivatives of piperine, an alkaloid from the pepper plant Piper nigrum, for possible use in Parkinson's disease. Bioorg Med Chem Lett 22: 7183-8 (2012)
 US9144538, Piperine US9138393, Piperine BDBM181116
US9144538, Piperine US9138393, Piperine BDBM181116 N-[(E,E)-piperoyl]piperidine 1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)penta-2,4-dienoyl]piperidine CHEMBL43185 piperine BDBM50148573 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-2,4-pentadienoyl]piperidine (E,E)-1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine
N-[(E,E)-piperoyl]piperidine 1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)penta-2,4-dienoyl]piperidine CHEMBL43185 piperine BDBM50148573 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-2,4-pentadienoyl]piperidine (E,E)-1-piperoylpiperidine 1-[(2E,4E)-5-(1,3-benzodioxol-5-yl)-1-oxo-2,4-pentadienyl]piperidine