 BDBM433323 US20240140952, Compound (rac)-Ruxolitinib US10617692, Ruxolitinib US11414413, Example Ruxolitinib US10561657, Ruxolitinib
BDBM433323 US20240140952, Compound (rac)-Ruxolitinib US10617692, Ruxolitinib US11414413, Example Ruxolitinib US10561657, Ruxolitinib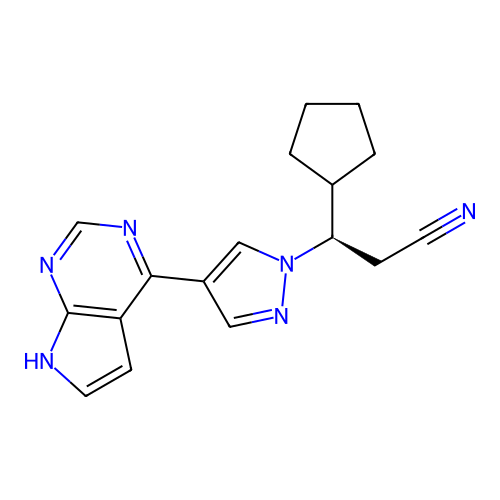 RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi
RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi
INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi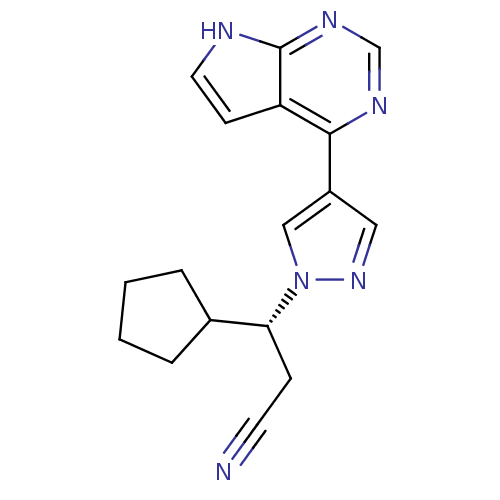 RUXOLITINIB PHOSPHATE INCB-018424 US10766894, Compound TABLE 1.1 US10875847, Compound JAKAFI US10112907, Example 00016 US11279703, TABLE 6.147 BDBM50355501 RUXOLITINIB US11203595, TABLE 1.1 US20240140952, Compound Ruxolitinib
RUXOLITINIB PHOSPHATE INCB-018424 US10766894, Compound TABLE 1.1 US10875847, Compound JAKAFI US10112907, Example 00016 US11279703, TABLE 6.147 BDBM50355501 RUXOLITINIB US11203595, TABLE 1.1 US20240140952, Compound Ruxolitinib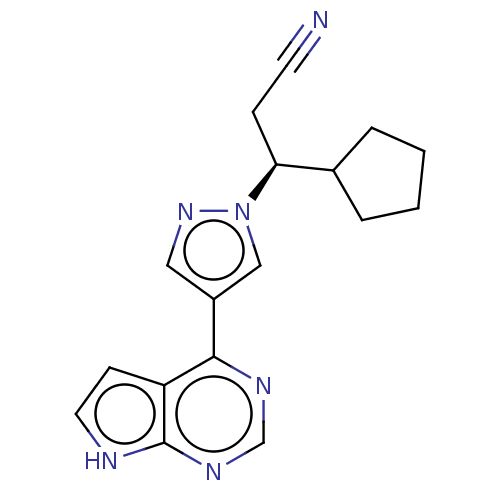 US20240140952, Compound (S)-Ruxolitinib CHEMBL1287854 BDBM50557386
US20240140952, Compound (S)-Ruxolitinib CHEMBL1287854 BDBM50557386
- Davis, RR; Li, B; Yun, SY; Chan, A; Nareddy, P; Gunawan, S; Ayaz, M; Lawrence, HR; Reuther, GW; Lawrence, NJ; Schönbrunn, E Structural Insights into JAK2 Inhibition by Ruxolitinib, Fedratinib, and Derivatives Thereof. J Med Chem 64: 2228-2241 (2021)
- Yao, L; Ramanujulu, PM; Poulsen, A; Ohlson, S; Dymock, BW Merging of ruxolitinib and vorinostat leads to highly potent inhibitors of JAK2 and histone deacetylase 6 (HDAC6). Bioorg Med Chem Lett 28: 2636-2640 (2018)
- Yao, L; Mustafa, N; Tan, EC; Poulsen, A; Singh, P; Duong-Thi, MD; Lee, JXT; Ramanujulu, PM; Chng, WJ; Yen, JJY; Ohlson, S; Dymock, BW Design and Synthesis of Ligand Efficient Dual Inhibitors of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Based on Ruxolitinib and Vorinostat. J Med Chem 60: 8336-8357 (2017)
- Biochemical JAK Kinase Assay In some embodiments, the additional pharmaceutical agent is a JAK1 and/or JAK2 inhibitor. In some embodiments, the present application provides a method of treating a disease described herein (e.g., a B cell malignancy, such as diffuse B-cell lymphoma) in a patient comprising administering to the patient a compound described herein, or a pharmaceutically acceptable salt thereof, and a JAK1 and/or JAK2 inhibitor. The B cell malignancies can include those described herein and in U.S. Ser. No. 61/976,815, filed Apr. 8, 2014. In some embodiments, the inhibitor of JAK1 and/or JAK2 is 3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile. In some embodiments, the inhibitor of JAK1 and/or JAK2 is (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile (ruxolitinib; also known as INCB018424). Ruxolitinib has an IC50 of less than 10 nM at 1 mM ATP (assay J) at JAK1 and JAK2. 3-Cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile and ruxolitinib can be made by the procedure described in U.S. Pat. No. 7,598,257 (Example 67), filed Dec. 12, 2006, which is incorporated herein by reference in its entirety. In some embodiments, the inhibitor of JAK1 and/or JAK2 is (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]propanenitrile phosphoric acid salt.
- JAK/TYK2 Assay 10 mM test compound stock or 1 mM control compound stock (tofocitinib, ruxolitinib or staurosporine) in DMSO was diluted to 0.4 mM in DMSO. A 3-fold series dilution was then performed in DMSO to generate 10 different compound concentrations. The assay was carried out in 384-well white plate. 0.5 uL of 40× compound DMSO solution at different concentrations was mixed with 10 uL 2× enzyme prepared in reaction buffer (20 mM HEPES, 10 mM MgCl2, 0.01% Tween, 1 mM DTT, pH 7.5). 10 uL 2× substrate mixture prepared in reaction buffer was then added to start the reaction. A short spin was done to settle down all solutions to the bottom of the plate. Final concentrations of test compound in the reaction mixture were 10000, 3333, 1111, 370, 123, 41.2, 13.7, 4.57, 1.52 and 0.51 nM. Concentrations of control compound were ten times less. Enzymatic reaction was conducted at 25° C. for 1-2 hours. 10 uL of Kinase Glo Reagents was added to stop the reaction and generate the luminescent signal which was measured using Envision.
- JAK/TYK2 Assay 10 mM test compound stock or 1 mM control compound stock (tofocitinib, ruxolitinib or staurosporine) in DMSO was diluted to 0.4 mM in DMSO. A 3-fold series dilution was then performed in DMSO to generate 10 different compound concentrations. The assay was carried out in 384-well white plate. 0.5 uL of 40× compound DMSO solution at different concentrations was mixed with 10 uL 2× enzyme prepared in reaction buffer (20 mM HEPES, 10 mM MgCl2, 0.01% Tween, 1 mM DTT, pH 7.5). 10 uL 2× substrate mixture prepared in reaction buffer was then added to start the reaction. A short spin was done to settle down all solutions to the bottom of the plate. Final concentrations of test compound in the reaction mixture were 10000, 3333, 1111, 370, 123, 41.2, 13.7, 4.57, 1.52and 0.51 nM. Concentrations of control compound were ten times less. Enzymatic reaction was conducted at 25° C. for 1-2 hours. 10 uL of Kinase Glo Reagents was added to stop the reaction and generate the luminescent signal which was measured using Envision. Luminescence signal was inversely related to kinase activity. Reaction mixture which did not contain enzyme served as negative control. The mixture without any compound was the positive control.
- JAK/TYK2 Assay 10 mM test compound stock or 1 mM control compound stock (tofocitinib, ruxolitinib or staurosporine) in DMSO was diluted to 0.4 mM in DMSO. A 3-fold series dilution was then performed in DMSO to generate 10 different compound concentrations. The assay was carried out in 384-well white plate. 0.5 uL of 40× compound DMSO solution at different concentrations was mixed with 10 uL 2× enzyme prepared in reaction buffer (20 mM HEPES, 10 mM MgCl2, 0.01% Tween, 1 mM DTT, pH 7.5). 10 uL 2× substrate mixture prepared in reaction buffer was then added to start the reaction. A short spin was done to settle down all solutions to the bottom of the plate. Final concentrations of test compound in the reaction mixture were 10000, 3333, 1111, 370, 123, 41.2, 13.7, 4.57, 1.52 and 0.51 nM. Concentrations of control compound were ten times less. Enzymatic reaction was conducted at 25° C. for 1-2 hours. 10 uL of Kinase Glo Reagents was added to stop the reaction and generate the luminescent signal which was measured using Envision. Luminescence signal was inversely related to kinase activity. Reaction mixture which did not contain enzyme served as negative control. The mixture without any compound was the positive control. Final concentration of enzymes and substrates and incubation time are summarized in the table below.[enz] [ATP] [sub] timeJAK1 7.5 nM 2 uM 30 uM (IRS-1) 1 hrJAK2 0.8 nM 2 uM 4 uM (pEY) 1 hrJAK3 1.5 nM 2 uM 4 uM (pEY) 1 hrTYK2 9 nM 2 uM 30 uM (IRS-1) 1 hr
 BDBM433323 US20240140952, Compound (rac)-Ruxolitinib US10617692, Ruxolitinib US11414413, Example Ruxolitinib US10561657, Ruxolitinib
BDBM433323 US20240140952, Compound (rac)-Ruxolitinib US10617692, Ruxolitinib US11414413, Example Ruxolitinib US10561657, Ruxolitinib RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi
RUXOLITINIB PHOSPHATE BDBM50391992 US20250136608, Compound Ruxolitinib INCB018424 SALT Jakafi INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi
INCB-18424 PHOSPHATE INCB018424 PHOSPHATE Ruxolitinib (as phosphate) Ruxolitinib phosphate BDBM50649917 INCB-018424 SALT Opzelura Ruxolitinib monophosphate INCB-018424 PHOSPHATE INCB018424 SALT Jakafi Jakavi RUXOLITINIB PHOSPHATE INCB-018424 US10766894, Compound TABLE 1.1 US10875847, Compound JAKAFI US10112907, Example 00016 US11279703, TABLE 6.147 BDBM50355501 RUXOLITINIB US11203595, TABLE 1.1 US20240140952, Compound Ruxolitinib
RUXOLITINIB PHOSPHATE INCB-018424 US10766894, Compound TABLE 1.1 US10875847, Compound JAKAFI US10112907, Example 00016 US11279703, TABLE 6.147 BDBM50355501 RUXOLITINIB US11203595, TABLE 1.1 US20240140952, Compound Ruxolitinib US20240140952, Compound (S)-Ruxolitinib CHEMBL1287854 BDBM50557386
US20240140952, Compound (S)-Ruxolitinib CHEMBL1287854 BDBM50557386