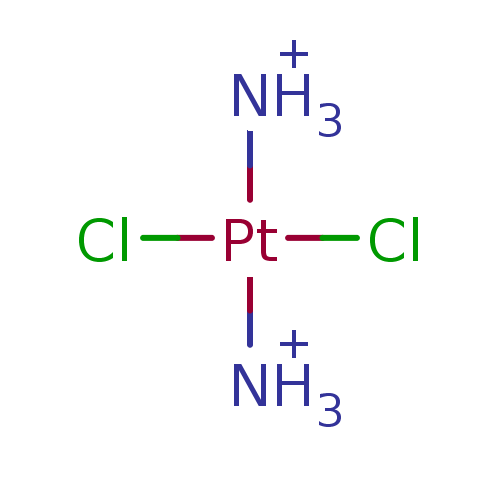 BDBM92386 trans-DDP, 8 Cisplatin, 1
BDBM92386 trans-DDP, 8 Cisplatin, 1 Cis-Platinum(II) trans-dichlorodiammineplatinum.(transplatin) camphorato platinum complex derivative cis-Platinum diamminedichloride Platinum(IV) Complex Pt(II) Complex cis-diamminedichloroplatinum(II)(cis-DDP) Cisplatin(cis-Diammenedichloroplatinum) dichloromethanediamine(platinum complex) cisplatin complex dichloroplatinumdiamine cisplatin US11952364, Example Control BDBM50028111 Platinum (II) complex
Cis-Platinum(II) trans-dichlorodiammineplatinum.(transplatin) camphorato platinum complex derivative cis-Platinum diamminedichloride Platinum(IV) Complex Pt(II) Complex cis-diamminedichloroplatinum(II)(cis-DDP) Cisplatin(cis-Diammenedichloroplatinum) dichloromethanediamine(platinum complex) cisplatin complex dichloroplatinumdiamine cisplatin US11952364, Example Control BDBM50028111 Platinum (II) complex
- Pflieger, M; Hamacher, A; Öz, T; Horstick-Muche, N; Boesen, B; Schrenk, C; Kassack, MU; Kurz, T Novel α,β-unsaturated hydroxamic acid derivatives overcome cisplatin resistance. Bioorg Med Chem 27: (2019)
- PubChem, PC SAR analysis of compounds that potentiate TRAIL-induced apoptosis in PPC-1 cells. PubChem Bioassay (2009)
- Zhang, L; Zheng, Y; Callahan, B; Belfort, M; Liu, Y Cisplatin inhibits protein splicing, suggesting inteins as therapeutic targets in mycobacteria. J Biol Chem 286: 1277-82 (2011)
- PubChem, PC SAR analysis of compounds that potentiate TRAIL-induced apoptosis in MDA-MB-435 cells. PubChem Bioassay (2009)
- Wei, H; Gou, W; Gao, J; Ning, H; Song, Y; Li, D; Qin, Y; Hou, W; Li, Y Novel PHD2/HDACs hybrid inhibitors protect against cisplatin-induced acute kidney injury. Eur J Med Chem 230: (2022)
- Stenzel, K; Hamacher, A; Hansen, FK; Gertzen, CGW; Senger, J; Marquardt, V; Marek, L; Marek, M; Romier, C; Remke, M; Jung, M; Gohlke, H; Kassack, MU; Kurz, T Alkoxyurea-Based Histone Deacetylase Inhibitors Increase Cisplatin Potency in Chemoresistant Cancer Cell Lines. J Med Chem 60: 5334-5348 (2017)
- Asfaha, Y; Schrenk, C; Alves Avelar, LA; Lange, F; Wang, C; Bandolik, JJ; Hamacher, A; Kassack, MU; Kurz, T Novel alkoxyamide-based histone deacetylase inhibitors reverse cisplatin resistance in chemoresistant cancer cells. Bioorg Med Chem 28: (2020)
- Hazlitt, RA; Teitz, T; Bonga, JD; Fang, J; Diao, S; Iconaru, L; Yang, L; Goktug, AN; Currier, DG; Chen, T; Rankovic, Z; Min, J; Zuo, J Development of Second-Generation CDK2 Inhibitors for the Prevention of Cisplatin-Induced Hearing Loss. J Med Chem 61: 7700-7709 (2018)
- Zang, YD; Wu, HJ; Chen, XY; Ma, ZL; Li, CJ; Ma, J; Chen, XG; Sheng, L; Zhang, S; Zhang, DM Synthesis and Biological Evaluation of Novel Psidium Meroterpenoid Derivatives against Cisplatin-Induced Acute Kidney Injury. J Med Chem 67: 14234-14255
- Cui, H; Hu, Z; Yang, K; Huang, J; Wu, Y; Chen, Q; Wei, R; Wang, P; Wang, H; Li, H; Chen, Y; Lu, T; Yao, Y; Zhu, Y Design and synthesis of highly TRAIL expression HDAC inhibitors based on ONC201 to promote apoptosis of colorectal cancer. Eur J Med Chem 238: (2022)
- PubChem, PC Dose Response confirmation of uHTS hits for a small molecule Caspase-8 TRAIL sensitizers in a luminescence panel assay PubChem Bioassay (2013)
- Horley, NJ; Beresford, KJ; Chawla, T; McCann, GJ; Ruparelia, KC; Gatchie, L; Sonawane, VR; Williams, IS; Tan, HL; Joshi, P; Bharate, SS; Kumar, V; Bharate, SB; Chaudhuri, B Discovery and characterization of novel CYP1B1 inhibitors based on heterocyclic chalcones: Overcoming cisplatin resistance in CYP1B1-overexpressing lines. Eur J Med Chem 129: 159-174 (2017)
- Shin, SY; Jung, H; Ahn, S; Hwang, D; Yoon, H; Hyun, J; Yong, Y; Cho, HJ; Koh, D; Lee, YH; Lim, Y Polyphenols bearing cinnamaldehyde scaffold showing cell growth inhibitory effects on the cisplatin-resistant A2780/Cis ovarian cancer cells. Bioorg Med Chem 22: 1809-20 (2014)
- Wang, CH; Wu, HT; Cheng, HM; Yen, TJ; Lu, IH; Chang, HC; Jao, SC; Shing, TK; Li, WS Inhibition of glutathione S-transferase M1 by new gabosine analogues is essential for overcoming cisplatin resistance in lung cancer cells. J Med Chem 54: 8574-81 (2011)
- Huo, Z; Liu, K; Zhang, X; Liang, Y; Sun, X Discovery of pyrimidine-bridged CA-4 CBSIs for the treatment of cervical cancer in combination with cisplatin with significantly reduced nephrotoxicity. Eur J Med Chem 235: (2022)
- Le, TV; Suh, JH; Kim, N; Park, HJ In silico identification of poly(ADP-ribose)polymerase-1 inhibitors and their chemosensitizing effects against cisplatin-resistant human gastric cancer cells. Bioorg Med Chem Lett 23: 2642-6 (2013)
- Huang, W; Liu, Y; Wang, J; Yuan, X; Jin, HW; Zhang, LR; Zhang, JT; Liu, ZM; Cui, JR Small-molecule compounds targeting the STAT3 DNA-binding domain suppress survival of cisplatin-resistant human ovarian cancer cells by inducing apoptosis. Eur J Med Chem 157: 887-897 (2018)
- Williams, IS; Joshi, P; Gatchie, L; Sharma, M; Satti, NK; Vishwakarma, RA; Chaudhuri, B; Bharate, SB Synthesis and biological evaluation of pyrrole-based chalcones as CYP1 enzyme inhibitors, for possible prevention of cancer and overcoming cisplatin resistance. Bioorg Med Chem Lett 27: 3683-3687 (2017)
- Cheng, JH; Huang, AM; Hour, TC; Yang, SC; Pu, YS; Lin, CN Antioxidant xanthone derivatives induce cell cycle arrest and apoptosis and enhance cell death induced by cisplatin in NTUB1 cells associated with ROS. Eur J Med Chem 46: 1222-31 (2011)
- Sharma, R; Gatchie, L; Williams, IS; Jain, SK; Vishwakarma, RA; Chaudhuri, B; Bharate, SB Glycyrrhiza glabra extract and quercetin reverses cisplatin resistance in triple-negative MDA-MB-468 breast cancer cells via inhibition of cytochrome P450 1B1 enzyme. Bioorg Med Chem Lett 27: 5400-5403 (2017)
- Endo, S; Xia, S; Suyama, M; Morikawa, Y; Oguri, H; Hu, D; Ao, Y; Takahara, S; Horino, Y; Hayakawa, Y; Watanabe, Y; Gouda, H; Hara, A; Kuwata, K; Toyooka, N; Matsunaga, T; Ikari, A Synthesis of Potent and Selective Inhibitors of Aldo-Keto Reductase 1B10 and Their Efficacy against Proliferation, Metastasis, and Cisplatin Resistance of Lung Cancer Cells. J Med Chem 60: 8441-8455 (2017)
- Bromidge, SM; Davies, S; Duckworth, DM; Forbes, IT; Jones, GE; Jones, J; King, FD; Blackburn, TP; Holland, V; Kennett, GA; Lightowler, S; Middlemiss, DN; Riley, GJ; Trail, B; Wood, MD Bioorg Med Chem Lett 10: 1867-70 (2000)
- Heightman, TD; Gaster, LM; Pardoe, SL; Pilleux, JP; Hadley, MS; Middlemiss, DN; Price, GW; Roberts, C; Scott, CM; Watson, JM; Gordon, LJ; Holland, VA; Powles, J; Riley, GJ; Stean, TO; Trail, BK; Upton, N; Austin, NE; Ayrton, AD; Coleman, T; Cutler, L Bioorg Med Chem Lett 15: 4370-4 (2005)
- Wilhelm, SM; Carter, C; Tang, L; Wilkie, D; McNabola, A; Rong, H; Chen, C; Zhang, X; Vincent, P; McHugh, M; Cao, Y; Shujath, J; Gawlak, S; Eveleigh, D; Rowley, B; Liu, L; Adnane, L; Lynch, M; Auclair, D; Taylor, I; Gedrich, R; Voznesensky, A; Riedl, B; Post, LE; Bollag, G; Trail, PA Cancer Res 64: 7099-109 (2004)
- ChEMBL_2323985 Inhibition of ABCC1 (unknown origin) overexpressing human KBV cells mediated efflux assessed as cisplatin IC50 using cisplatin as substrate at 5 uM
- ChEMBL_2323986 Inhibition of ABCC1 (unknown origin) overexpressing human KBV cells mediated efflux assessed as cisplatin IC50 using cisplatin as substrate at 10 uM
- ChEMBL_2323987 Inhibition of ABCC1 (unknown origin) overexpressing human KBV cells mediated efflux assessed as cisplatin IC50 using cisplatin as substrate at 25 uM
- ChEMBL_2114436 (CHEMBL4823377) Inhibition of HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 0.7 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_2114440 (CHEMBL4823381) Inhibition of HDAC in human CAL-27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 0.7 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_1838326 (CHEMBL4338459) Inhibition of class 1 HDAC in human CAL27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 5 uM preincubated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 9.79 uM)
- ChEMBL_1838330 (CHEMBL4338463) Inhibition of class 1 HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 5 uM preincubated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 63.2 uM)
- ChEMBL_1838431 (CHEMBL4338564) Inhibition of class 1 HDAC in human CAL27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 0.5 uM preincubated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 9.79 uM)
- ChEMBL_1838432 (CHEMBL4338565) Inhibition of class 1 HDAC in human CAL27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 3.2 uM preincubated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 9.79 uM)
- ChEMBL_1838433 (CHEMBL4338566) Inhibition of class 1 HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 0.5 uM preincubated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 63.2 uM)
- ChEMBL_1838434 (CHEMBL4338567) Inhibition of class 1 HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 3.2 uM preincubated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 63.2 uM)
- ChEMBL_2114437 (CHEMBL4823378) Inhibition of HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 3 to 4 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_2114438 (CHEMBL4823379) Inhibition of HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 2 to 3 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_2114439 (CHEMBL4823380) Inhibition of HDAC in human Cal27CisR cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 0.3 to 0.5 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_2114441 (CHEMBL4823382) Inhibition of HDAC in human CAL-27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 3 to 4 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_2114442 (CHEMBL4823383) Inhibition of HDAC in human CAL-27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 2 to 3 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_2114443 (CHEMBL4823384) Inhibition of HDAC in human CAL-27 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 0.3 to 0.5 uM pretreated for 48 hrs followed by cisplatin addition and measured after 72 hrs by MTT assay (Rvb = 3.57 uM)
- ChEMBL_1747469 (CHEMBL4181979) Inhibition of recombinant human CYP1B1 expressed in HEK293 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 by MTT assay (Rvb = 65.01 +/- 7.01 uM)
- ChEMBL_1751562 (CHEMBL4186322) Inhibition of human CYP1B1 expressed in MDA-MB-468 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 6.6 uM (Rvb = 6.70+/- 0.7 uM)
- ChEMBL_1751563 (CHEMBL4186323) Inhibition of human CYP1B1 expressed in MDA-MB-468 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 20 uM (Rvb = 6.70+/- 0.7 uM)
- ChEMBL_1659850 (CHEMBL4009462) Inhibition of human CYP1B1 expressed in HEK293 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 0.02 uM by MTT assay (Rvb = 61 +/- 8 uM)
- ChEMBL_1659851 (CHEMBL4009463) Inhibition of human CYP1B1 expressed in HEK293 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 0.018 uM by MTT assay (Rvb = 61 +/- 8 uM)
- ChEMBL_1659852 (CHEMBL4009464) Inhibition of human CYP1B1 expressed in HEK293 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 0.016 uM by MTT assay (Rvb = 61 +/- 8 uM)
- ChEMBL_1659841 (CHEMBL4009453) Inhibition of human CYP1B1 expressed in human A2780 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 0.02 uM by MTT assay (Rvb = 40 +/- 4.9 uM)
- ChEMBL_1659842 (CHEMBL4009454) Inhibition of human CYP1B1 expressed in human A2780 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 0.018 uM by MTT assay (Rvb = 40 +/- 4.9 uM)
- ChEMBL_1659843 (CHEMBL4009455) Inhibition of human CYP1B1 expressed in human A2780 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin EC50 at 0.016 uM by MTT assay (Rvb = 40 +/- 4.9 uM)
- ChEMBL_2083515 (CHEMBL4739306) Reversal of MRP2-mediated multidrug resistance in human KBV cells assessed as cisplatin IC50 at 5 uM after 72 hrs in presence of cisplatin by MTT assay (Rvb = 4902.89 +/- 130.33 nM)
- ChEMBL_2083516 (CHEMBL4739307) Reversal of MRP2-mediated multidrug resistance in human KBV cells assessed as cisplatin IC50 at 10 uM after 72 hrs in presence of cisplatin by MTT assay (Rvb = 4902.89 +/- 130.33 nM)
- ChEMBL_1741911 (CHEMBL4157661) Inhibition of ABCB1 in human SW620/Ad300 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 0.61 +/- 0.03 microM)
- ChEMBL_1741912 (CHEMBL4157662) Inhibition of ABCB1 in human SW620/Ad300 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 20 uM after 72 hrs by MTT assay (Rvb = 0.61 +/- 0.03 microM)
- ChEMBL_1741913 (CHEMBL4157663) Inhibition of ABCB1 in human SW620/Ad300 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 4 uM after 72 hrs by MTT assay (Rvb = 0.61 +/- 0.03 microM)
- ChEMBL_1852720 (CHEMBL4353344) Inhibition of P-gp in HEK293/ABCB1 cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 2 uM incubated for 48 hrs by MTT assay (Rvb = 8.08 uM)
- ChEMBL_1741947 (CHEMBL4157697) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 10 uM after 72 hrs by MTT assay (Rvb = 7.22 +/- 0.45 microM)
- ChEMBL_1741948 (CHEMBL4157698) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 20 uM after 72 hrs by MTT assay (Rvb = 7.22 +/- 0.45 microM)
- ChEMBL_1741949 (CHEMBL4157699) Inhibition of ABCB1 (unknown origin) expressed in HEK293T cells assessed as potentiation of cisplatin-induced cytotoxicity by measuring cisplatin IC50 at 4 uM after 72 hrs by MTT assay (Rvb = 7.22 +/- 0.45 microM)
- SAR analysis of compounds that potentiate TRAIL-induced apoptosis in MDA-MB-435 cells. Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Number: X01 MH083230-01 Assay Provider: Dr. Dmitri Rozanov, Sanford-Burnham Medical Research Institute, San Diego CA This assay was developed and performed to confirm hits originally identified in "uHTS for the identification of compounds that potentiate TRAIL-induced apoptosis of cancer cells" (AID 1443) and to study the structure-activity relationship on analogs of the confirmed hits. Compounds are either acquired from commercial sources or synthesized internally. The TRAIL-resistant cell line, MDA-MB-435 is used, because we would like to determine if compounds can potentiate TRAIL-mediated cytotoxicity not only in TRAIL-sensitive PPC-1 carcinoma cells(AIDs 1443 and 1624) but also in TRAIL-resistant cells. Cytotoxic chemotherapy induces apoptosis via a pat
- ChEMBL_1706498 (CHEMBL4057731) Inhibition of human CYP1B1 transfected in HEK293 cells assessed as cisplatin EC50 at 6 times IC50 by MTT assay (Rvb = 65 uM)
- ChEMBL_1670186 (CHEMBL4020074) Inhibition of full length human XPA expressed in Sf9 cells assessed as reduction in interaction of XPA with 32P-labeled cisplatin-modified dsDNA by EMSA
- Fluorescent Reporter Assay In vitro inhibition of the Mtu RecA Intein by Cisplatin-Splicing activity of the RecA intein was dertermined in the presence and absence of potential platinum (II) inhibitors using a fluorescent reporter assay.
- Biological Assay 5000 PPC-1 cells were plated and grown overnight. Compounds were plated and 4 hrs later, TRAIL was added to half of the plate while RPMI was added to the other half of the plate as a control. Plates were return to the incubator for 24 hrs. Plates were removed from the incubator and placed on the bench for 30 min and then 25 uL of Cell Titer Glo were added per well. Plates were placed on a rocker and then read on a luminometer. 5000 MDA-MB-231 cells were plated per well. Compound was added and 4 hrs later, TRAIL was added at 5 ng/mL; RPMI was added for a minus TRAIL control. Plates were incubated an additional 24 hrs, removed to the bench for 30 min, and then 25 uL of cell titer glo was added per well. Plates were placed on a rocker and read on a luminometer. Data were fit using PRISM.
- Biological Assays 5000 PPC-1 cells were plated and grown overnight. Compounds were plated and 4 hrs later, TRAIL was added to half of the plate while RPMI was added to the other half of the plate as a control. Plates were return to the incubator for 24 hrs. Plates were removed from the incubator and placed on the bench for 30 min and then 25 uL of Cell Titer Glo were added per well. Plates were placed on a rocker and then read on a luminometer. 5000 MDA-MB-231 cells were plated per well. Compound was added and 4 hrs later, TRAIL was added at 5 ng/mL; RPMI was added for a minus TRAIL control. Plates were incubated an additional 24 hrs, removed to the bench for 30 min. and then 25 uL of cell titer glo was added per well. Plates were placed on a rocker and read on a luminometer. Data were fit using PRISM.
- SAR analysis of compounds that potentiate TRAIL-induced apoptosis in PPC-1 cells. Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, CA) Network: NIH Molecular Libraries Production Centers Network (MLPCN) Grant Number: X01 MH083230-01 Assay Provider: Dr. Dmitri Rozanov, Sanford-Burnham Medical Research Institute, San Diego CA This dose response assay is developed and performed to confirm hits originally identified in "uHTS for the identification of compounds that potentiate TRAIL-induced apoptosis of cancer cells" (AID 1443) and to study the structure-activity relationship on analogs of the confirmed hits. Compounds are either acquired from commercial sources or synthesized internally. Cytotoxic chemotherapy induces apoptosis via a pathway involving mitochondria, sometimes referred to as the "intrinsic pathway." An acquired resistance to anticancer drugs commonly results from the accumulation of defects in components of the mitochondrial pathway for apoptosis. Discov
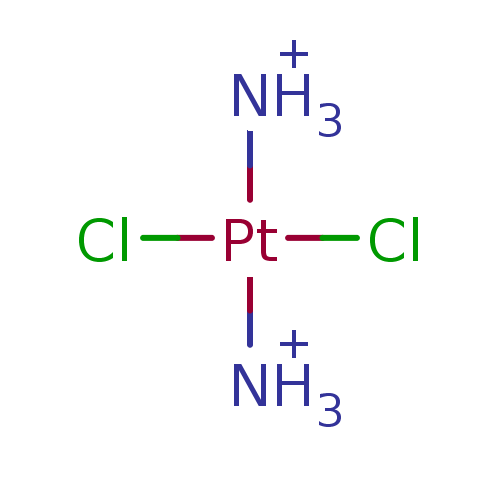 BDBM92386 trans-DDP, 8 Cisplatin, 1
BDBM92386 trans-DDP, 8 Cisplatin, 1 Cis-Platinum(II) trans-dichlorodiammineplatinum.(transplatin) camphorato platinum complex derivative cis-Platinum diamminedichloride Platinum(IV) Complex Pt(II) Complex cis-diamminedichloroplatinum(II)(cis-DDP) Cisplatin(cis-Diammenedichloroplatinum) dichloromethanediamine(platinum complex) cisplatin complex dichloroplatinumdiamine cisplatin US11952364, Example Control BDBM50028111 Platinum (II) complex
Cis-Platinum(II) trans-dichlorodiammineplatinum.(transplatin) camphorato platinum complex derivative cis-Platinum diamminedichloride Platinum(IV) Complex Pt(II) Complex cis-diamminedichloroplatinum(II)(cis-DDP) Cisplatin(cis-Diammenedichloroplatinum) dichloromethanediamine(platinum complex) cisplatin complex dichloroplatinumdiamine cisplatin US11952364, Example Control BDBM50028111 Platinum (II) complex