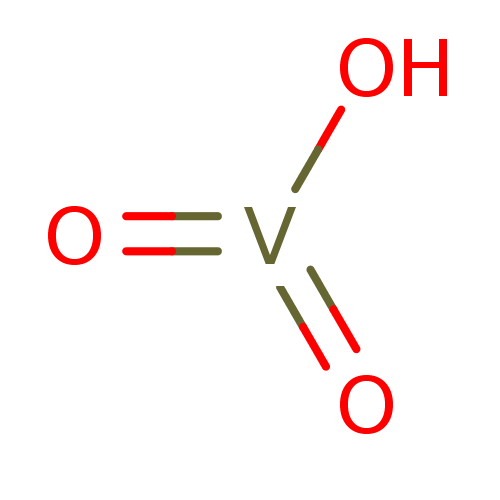
 Vanadate CHEMBL86473 BDBM50094936
Vanadate CHEMBL86473 BDBM50094936 Sodium vanadate CHEMBL294467 BDBM50115732
Sodium vanadate CHEMBL294467 BDBM50115732
- ChEMBL_1625446 (CHEMBL3867915) Binding affinity to Vanadate-sensitive ABCB1 (unknown origin)
- ChEMBL_1932786 (CHEMBL4478438) Inhibition of human ABCG2 expressed in baculovirus infected Sf9 cell membrane assessed as inhibition of vanadate sensitive basal ATPase activity after 20 mins by colorimetric method
- ChEMBL_1932787 (CHEMBL4478439) Inhibition of human ABCG2 expressed in baculovirus infected Sf9 cell membrane assessed as inhibition of vanadate sensitive quercetin-stimulated ATPase activity after 20 mins by colorimetric method
- ChEMBL_2129308 (CHEMBL4838737) Inhibition of MERTK in human 697 cells assessed as reduction in phosphorylation of MERTK preincubated for 1 hr followed by vanadate addition and measured after 3 mins by immunoblot analysis
- ChEMBL_2129309 (CHEMBL4838738) Inhibition of FLT3 in human SEM cells assessed as reduction in phosphorylation of MERTK preincubated for 1 hr followed by vanadate addition and measured after 3 mins by immunoblot analysis
- ChEMBL_760655 (CHEMBL1815640) Inhibition of human MRP1 expressed in Spodoptera frugiperda Sf9 cells assessed as inhibition of NEM-GS-induced vanadate-sensitive ATPase activity measured by generation of inorganic phosphate by spectrophotometry in presence of glutathione
- TrkA Enzyme Activity Test In a 10 μL reaction system with a buffer of 20 mM Hepes (pH 7.5), 10 mM MgCl2, 1 mM EGTA, 0.01% Brij35, 0.1 mM sodium vanadate, 0.02 g/mL BSA, 2 mM DTT, 1% DMSO medium, containing 15 nM TrkA kinase, 0.3 μM biotin-TK peptide (biotin-labeled tyrosine kinase substrate polypeptide), 100 μM ATP were incubated at 23° C. for 120 minutes. The reaction was spotted on P81 ion exchange paper (Whatman #3698-915), the filter was washed thoroughly with 0.75% phosphoric acid, and the radiophosphorylated substrate remaining on the filter was measured. Kinase activity data were expressed as a percentage of kinase activity in the test sample compared to the vehicle (DMSO) reaction. IC50 and curve fitting can be obtained by Graphpad software Prism4.
- CDK2/CycE Kinase Assay Recombinant fusion proteins of GST and human CDK2 and of GST and human CycE, expressed in insect cells (Sf9) and purified by Glutathion-Sepharose affinity chromatography, were purchased from ProQinase GmbH (Freiburg, Germany). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI Peptide Technologies (Berlin, Germany)For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK2/CycE in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22 C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction.
- CDK9/CycT1 Recombinant full-length His-tagged human CDK9 and CycT1, expressed in insect cells and purified by Ni-NTA affinity chromatography, were purchase from Invitrogen (Cat. No PV4131). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI peptide technologies (Berlin, Germany) For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction.
- CDK9/CycT1 Kinase Assay Recombinant full-length His-tagged human CDK9 and CycT1, expressed in insect cells and purified by Ni-NTA affinity chromatography, were purchased from Invitrogen (Cat. No PV4131). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI Peptide Technologies (Berlin, Germany) For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22 C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction.
- Kinase Assay CDK1/CycB (200 ng/measuring point) was incubated for 10 min at 22 C. in the presence of different concentrations of test substances (0 and within the range 0.001-10 uM) in assay buffer [50 mM Tris/HCl pH 8.0; 10 mM MgCl2; 0.1 mM Na ortho-vanadate; 1.0 mM dithiothreitol; 0.5 uM adenosine trisphosphate (ATP); 10 ug/measuring point histone IIIS; 0.2 uCi/measuring point 33P-gamma ATP; 0.05% NP40; 1.25% dimethyl sulphoxide]. The reaction was stopped by adding EDTA solution (250 mM; pH 8.0; 15 ul/measuring point).From each reaction mixture, 15 ul were applied to P30 filter strips (Wallac), and unincorporated 33P-ATP was removed by washing the filter strips three times, for 10 min in each case, in 0.5% phosphoric acid. After drying the filter strips for 1 hour at 70 C, the filter strips were covered with scintillator strips (MeltiLex A, Wallac) and stoved for 1 hour at 90 C. The amount of incorporated 33P (substrate phosphorylation) was determined.
- Kinase Assay Recombinant CDK4 and CycD1-GST fusion proteins, purified from baculovirus-infected insect cells (Sf9), were purchased from ProQinase GmbH, Freiburg. CDK4/CycD1 (250 ng/measuring point) was incubated for 3 hours at 22 C. in the presence of different concentrations of test substances (0 uM, and within the range 0.001-10 uM) in 31 ul of assay buffer [50 mM Hepes pH 7.0; 2.5 mM MnCl; 0.05 mM Na ortho-vanadate; 1.0 mM dithiothreitol; 0.25 uM adenosine trisphosphate (ATP); 0.5 uM biotinylated myelin basic protein (bio-MPB, GE Healthcare); 0.05 uCi/measuring point 33P-gamma ATP; 0.005% NP40; 0.025% bovine serum albumin; 3% dimethyl sulphoxide]. The reaction was stopped by adding 50 ul of stop-mix [100 uM ATP; 10 mM EDTA pH 8.0; 0.2% Triton X100; 0.125 mg of streptavidin-SPA Beads (GE Healthcare)]. After incubation for 10 min at room temperature, the SPA beads were pelleted by centrifugation (10 min; 1500 g).
- Kinase Assay VEGF receptor tyrosine kinase (90 ng/measuring point) was incubated for 10 min at 22 C. in the presence of different concentrations of test substances (0 uM, and within the range 0.001-10 M) in 30 ul of assay buffer [40 mM Tris/HCl pH 5.5; 10 mM MgCl2; 1 mM MnCl2; 3 uM Na ortho-vanadate; 1.0 mM dithiothreitol; 8 uM adenosine trisphosphate (ATP); 0.96 ug/measuring point poly-(Glu4Tyr); 0.2 uCi/measuring point 33P-gamma ATP; 1.4% dimethyl sulphoxide]. The reaction was stopped by adding EDTA solution (250 mM; pH 8.0; 15 ul/measuring point).From each reaction mixture, 15 ul were applied to P30 filter strips (Wallac), and unincorporated 33P-ATP was removed by washing the filter strips three times, for 10 min in each case, in 0.5% phosphoric acid. After drying the filter strips for 1 hour at 70 C., the filter strips were covered with scintillator strips (MeltiLex A, Wallac) and stoved for 1 hour at 90 C.
- In Vitro Evaluation of the Inhibitory Activity Against ROCK Protein Kinase Assay buffer solution: 20 mM 4-hydroxyethylpiperazine ethanesulfonic acid (pH 7.5), 10 mM magnesium chloride, 1 mM ethylene glycol-bis-(2-aminoethyl)tetraacetic acid, 0.02% polyethylene glycol monododecyl ether, 0.02 mg/mL bovine serum albumin, 0.1 mM sodium vanadate, 2 mM dithiothreitol, 1% DMSO.Experimental Operation:The freshly prepared buffer solution was added to ROCK protein kinase substrate (Long S6 Kinase substrate peptide), at a concentration of 20 μM, then 1 nM ROCK protein kinase was added thereto and stirred evenly. Echo550 was used to add a series of DMSO dilutions containing the test compound (starting from 10 μM, serially diluted by 3 times), the solution was pre-incubated at room temperature for 20 minutes, then 33P-ATP (radiation intensity 10 μCi/μL) was added to initiate the reaction, and the reaction was performed at room temperature for two hours. Then the solution was filtered by P81 ion exchange paper (Whatman #3698-915) and washed with 0.75% phosphoric acid. The radiation intensity was determined by Filter-Binding method.
- Kinase Assay CDK2/CycE (50 mg/measuring point) was incubated for 10 min at 22 C. in the presence of different concentrations of test substances (0 uM, and within the range 0.001-10 uM) in assay buffer [50 mM Tris/HCl pH 8.0; 10 mM MgCl2; 0.1 mM Na ortho-vanadate; 1.0 mM dithiothreitol; 0.5 uM adenosine trisphosphate (ATP); 10 ug/measuring point histone IIIS; 0.2 uCi/measuring point 33P-gamma ATP; 0.05% NP40; 1.25% dimethyl sulphoxide]. The reaction was stopped by adding EDTA solution (250 mM; pH 8.0; 15 ul/measuring point).From each reaction mixture, 15 ul were applied to P30 filter strips (Wallac), and unincorporated 33P-ATP was removed by washing the filter strips three times, for 10 min in each case, in 0.5% phosphoric acid. After drying the filter strips for 1 hour at 70 C., the filter strips were covered with scintillator strips (MeltiLex A, Wallac) and stoved for 1 hour at 90 C. The amount of incorporated 33P (substrate phosphorylation) was determined.
- BTK Enzyme Activity Assay The specific experimental process of BTK enzyme activity test is as follows:Buffer: 20 mM hydroxyethylpiperazine ethylsulfuric acid (Hepes) (pH 7.5), 10 mM magnesium chloride, 1 mM ethylene glycol bisaminoethyl ether tetraacetic acid (EGTA), 0.02% polyoxyethylene lauryl ether (Brij35), 0.02 mg/mL BSA, 0.1 mM sodium vanadate (Na3VO4), 2 mM dithiothreitol (DTT), 1% DMSO, 200 μM adenosine triphosphate (ATP).1. Configuring the substrate in the newly prepared reaction buffer2. Adding the required cofactors to the above substrate solution3. Adding the kinase BTKC481S to the above substrate solution and mixing well4. Adding the compound dissolved in DMSO to the kinase reaction mixture throughEcho550 (Acoustic technology; nanoliter range), and incubating at room temperature for 20 minutes5. Adding 33P-ATP (with a specific activity of 10 μCi/μL) into the reaction mixture to initiate the reaction6. Incubating at room temperature for 2 hours7. Detection of radioactivity by filtration-binding method8. Kinase activity data represent the percentage of remaining kinase activity in the test sample compared to the vehicle (dimethyl sulfoxide) reaction. Using Prism (GraphPad software) to obtain IC50 values and fitting curves
- HCK Kinase Assay HCK kinase reaction (10 μL) containing 4 nM N-terminally GST-tagged HCK (75-526), purified from insect expression system, 5 μM Control AMC Substrate, 2 μM Src-Family Kinase R110 Substrate, and 50 μM ATP in kinase reaction buffer (40 mM Tris-HCl pH 7.5, 20 mM MgCl2, 0.1 mg/mL BSA, 1 mM MnCl2, 0.1 mM Sodium Vanadate), and test compound 1:3 serially-diluted starting at 1 μM were incubated at room temperature (22-25° C.) for 60 minutes in 384 well plate (Corning, Cat. No. 4514). The procedure of ProFluor Src-Family Kinase Assay (Promega, Cat. No. V1271) was then followed. To the reaction was added 5 μL Protease solution and the mixture was incubated for 60 minutes at room temperature (22-25° C.), followed by 5 μL Stabilizer solution. The fluorescence signal was read on an Envision multilabel plate reader (Perkin Elmer). The R110 was then read at an excitation wavelength of 485 nm and an emission wavelength of 530 nm. The AMC signal was read at an excitation wavelength of 355 nm and an emission wavelength of 460 nm.
- Kinase Assay For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and peptide substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot.
- ALK Kinase Inhibition Test The specific experimental process was as follows: a test compound was formulated into a 10 mM mother solution with DMSO, stored at −20° C. after sub-packaging; the mother solution was thawed before use, diluted to 1 mM with DMSO, and then diluted with 1× kinase buffer (containing 50 mM 4-hydroxyethyl piperazine ethanesulfonic acid pH 7.0, 0.02% NaN3, 0.01% BSA, 0.1 mM ortho-vanadate, 5 mM MgCl2, 1 mM DTT, 37.5 nM supplementary enzyme buffer) by 40 times. 4 μL of the diluted solution of the test compound and 2 μL of 0.4 ng/well ALK kinase solution (Thermo Scientific) were added into reaction wells in turn, incubated for 10 min at room temperature, then 4 μL of 0.7 μM biotin-labeled tyrosine kinase substrate and 5 μM ATP solution were added to start the kinase reaction. After the reaction, 5 μL of 43.75 nM streptavidin-labeled XL665 was added, the materials were evenly mixed and then 5 μL of 0.04 nM europium-labeled tyrosine kinase antibody detection solution was imeddiately added. After reacting at room temperature for 1 hour, a fluorescence signal was detected using a SpectraMax i3× instrument (excited at 320 nm, emitted at 665 nm, 615 nm).
- BTK Enzyme Activity Assay The specific assay process of BTK enzyme activity assay is as follows: Buffer: 20 mM 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid (Hepes) (pH 7.5), 10 mM magnesium chloride, 1 mM ethylenebis(oxyethylenenitrilo)tetraacetic acid (EGTA), 0.02% polyoxyethylene dodecyl ether (Brij35), 0.02 mg/mL BSA, 0.1 mM sodium vanadate (Na3VO4), 2 mM dithiothreitol (DTT), 1% DMSO, 200 μM adenosine triphosphate (ATP).1. The substrate was prepared in freshly prepared reaction buffer;2. The required cofactor was added to the above-mentioned substrate solution;3. The kinase BTKC481S was added to the above-mentioned substrate solution and mixed well;4. The compound dissolved in DMSO was added to the kinase reaction mixture by Echo550 (Acoustic technology; nanoliter range), and the mixture was incubated at room temperature for 20 minutes;5. 33P-ATP (specific radioactivity of 10 μCi/μL) was added to the reaction mixture to initiate the reaction;6. The mixture was incubated at room temperature for 2 hours;7. The radioactivity was detected by filter-binding method;8. Kinase activity data represented the percentage of remaining kinase activity in the assay sample compared to the vehicle (dimethyl sulfoxide) reaction. IC50 values and fitted curve were obtained using Prism (GraphPad software).
- ATR/ATRIP Enzymatic Assay Detection of ATR kinase activity utilized the AlphaScreen system to measure the phosphorylation of the substrate protein p53. Recombinant purified ATR/ATRIP (Eurofins cat #14-953) at a final concentration of 0.63 nM in assay buffer (50 mM Hepes pH 7.4, 0.1 mM vanadate, 0.5 mM DTT, 0.1 mM EGTA, 5 mM MnCl2, 0.01% Brij-30, 1% glycerol, 0.05% BSA) was mixed with compound serially diluted in 10% DMSO. The final DMSO concentration was 1.25%. A pre-mix of GST-tagged p53 (full length, Enzo Life Sciences cat # BML-FW9370) and adenosine 5′-triphosphate, ATP (Sigma-Aldrich cat #10519979001, Roche Diagnostic) in assay buffer was added to the enzyme:compound mix for a final concentration of 25 nM GST-p53 and 3 μM ATP. The reaction was allowed to proceed at room temperature for 1 hour then stopped by the addition of a pre-mix of phospho-p53 (Ser 15) antibody (New England Biolabs cat #9284S) at 1:3000 final dilution, 14.3 pg/mL glutathione donor beads (PerkinElmer Life Sciences cat #6765301) and 14.3 pg/mL protein A acceptor beads (PerkinElmer Life Sciences cat #670137) final bead concentration in buffer (60 mM EDTA in 50 mM Tris, pH 7.4 and 0.1% BSA). Plates were incubated at room temperature in the dark for 4 hours and read on a BMG Polarstar using AlphaScreen dedicated filters.
- Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and peptide substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 ul assay volume).
- Kinase Assay For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and peptide substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 ul assay volume). The reaction was stopped by the addition of 3 ul of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-Jugesheim, Germany].
- JAK2 Activity Inhibitory Assay In the kinase reactions, fused proteins (6His tag-fused hJAK2 kinase domain (aa808-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NTA agarose were used. The kinase reactions were initiated by the addition of the following solutions of (a) to (c) to 96-well half-area white plates (plates, Corning Incorporated 3642).(a) 5 gmol/L TK substrate-biotin (cisbio) diluted by kinase buffer (50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.0)), 0.02% sodium azide, 0.1 mmol/L sodium vanadate, 5 mmol/L magnesium chloride, 1 mmol/L dithiothreitol, 0.01% bovine serum albumin), 100 mol/L ATP, 250 nmol/L Supplement Enzymatic buffer (cisbio) solution: 10 μL/well(b) Test-article solution prepared by using kinase buffer containing 5% dimethylsulfoxide: 10 μL/well(c) 7 ng/mL hJAK2 enzyme diluted by kinase buffer: 30 μL/wellA well in which ATP was not added was set out as a blank well.Plates were let stand at room temperature for 10 minutes from starting reactions.To the plates were added 50 μL/well of a buffer for detection containing TK-Antibody-Cryptate (5 tests/50 μL) and streptoavidine-addition XL665 (62.5 nmol/L) reagent (50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.0), 20 mM EDTA, 800 mmol/L potassium fluoride, 0.1% bovine serum albumin).One hour after the addition of the buffer for detection, fluorescence counts of each well were measured by a fluorescence microplate reader. Specifically, fluorescence counts in 620 nm excited in 337 nm, and fluorescence counts in 665 nm excited by fluorescence in 620 nm were measured.Ratio of each well was calculated from measured fluorescence counts (fluorescence counts in 665 nm/fluorescence counts in 620 nm×10000).
- Kinase Assay Exon 20-Mutant-EGFR(D770_N771insSVD): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine tri phosphate (ATP, 3.33 mM=>final cone, in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final cone, in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 15 pg/μl. The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, a terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66 Tb cryptate PT66 Eu Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5).
- Kinase Assay Exon 20-Mutant-EGFR(V769_D770insASV): For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine tri phosphate (ATP, 3.33 mM=>final cone, in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final cone, in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 2.5 pg/μl. The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, an terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66 Tb cryptate PT66 Eu Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5).
- Mps-1 Kinase Assay For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μl assay volume is 10 μM) and peptide substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 μl assay volume). The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-J gesheim, Germany].
- WT-EGFR Kinase Assay For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μL of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine-tri-phosphate (ATP, 3.33 mM=>final conc. in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 7.6 μg/μL. The reaction was stopped by the addition of 3 μL of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, a terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66-Tb-cryptate PT66-Eu-Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5).
- Aurora B Enzyme Assay Aurora B (2 μM) was preactivated by equivalent concentration of GST-INCENP in 30 mM Tris-HCl pH 8.0, 0.4 mM ATP, 2 mM MgCl2, 0.1 mM EGTA, 0.1% BME (beta mercaptoethanol), 0.1 mM sodium vanadate, 10 mM DTT for 3 hours at 30° C. This solution was then dialysed for 5 hours against 50 mM Tris-HCl, pH 7.5, 270 mM sucrose, 150 mM NaCl, 0.1 mM EDTA, 0.1% BME, 1 mM benzamidine and 0.2 mM PMSF at 4° C. Aurora B/INCENP complex was aliquoted and frozen at −80° C. §Human INCENP (826-919) clone DU930 was received from University of Dundee, it is a GST N-terminal tagged protein. A final concentration of 2 nM of Aurora B/INCENP complex was added to the assay buffer (25 mM HEPES, 25 mM NaCl 0.0025% Tween-20, pH 7.2 0.015% BSA, 1 μM DTT). 3 μl of this solution was added to wells containing 0.1 μl of various concentrations of compound or DMSO vehicle in Greiner low volume 384 well black plate at room temperature for 30 mins. The reaction was initiated by the presence of 3 μl of substrate reagent containing 100 nM 5FAM-PKA-tide (GRTGRRNSI-NH2), 2 M ATP and 2 mM MgCl2 in assay buffer (25 mM HEPES, 25mM NaCl 0.0025% Tween-20, pH 7.2 0.015% BSA, 1 μM DTT) with a final DMSO level of 1.7%. The reaction was incubated for a further 120 mins at room temperature, and then terminated by the addition of 6 μl of a 1:500 dilution Progressive Binding Reagent solution (Part: R7287) in the manufacturers buffer A (Part: R7285) and manufacturers buffer B (Part R7286) and left to incubate for 120 mins at room temperature. The degree of phosphorylation of the 5FAM-PKA-tide (GRTGRRNSI-NH2) was measured using an Acquest plate reader (Molecular Devices, Sunnyvale, US) with excitation 485 nM, emission at 530 nM and using a 505 nmM dichroic lens.
- EGFR Kinase Assay For the assay 50 nL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μL of a solution of EGFR in aqueous assay buffer [50 mM Hepes/HCl pH 7.0, 1 mM MgCl2, 5 mM MnCl2, 0.5 mM activated sodium ortho-vanadate, 0.005% (v/v) Tween-20]were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μL assay volume is 10 μM) and substrate (1.67 μM=>final conc. in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 20 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration were about 3 U/ml. The reaction was stopped by the addition of 5 μl of a solution of HTRF detection reagents (0.1 μM streptavidine-XL665 [Cis Biointernational] and 1 nM PT66-Tb-Cryptate, an terbium-cryptate labelled anti-phospho-tyrosine antibody from Cis Biointernational [instead of the PT66-Tb-cryptate PT66-Eu-Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (80 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5).The resulting mixture was incubated 1 h at 22° C. to allow the binding of the biotinylated phosphorylated peptide to the streptavidine-XL665 and the PT66-Eu-Chelate. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the PT66-Tb-Cryptate to the streptavidine-XL665.
- SYK Assa SYK kinase assay is a standard kinase assay adapted to a 96 well plate format. This assay is performed in 96-well format for IC50 determination with 8 samples which represented 10 half log dilutions and a 40 μL reaction volume. The assay measures the incorporation of radiolabeled 33P γATP into an N-terminally biotinylated peptide substrate, derived from naturally occurring phosphoacceptor consensus sequence (Biotin-11aa DY*E). Phosphorylated products were detected upon termination of reactions with EDTA and the addition of Streptavidin coated beads. Representative results are in Table II above.Assay plates: 96-well MultiScreen 0.65 um filter plates (Millipore Cat. No.: MADVNOB10) Streptavidin coated beads: Streptavidin Sepharose , suspension 5.0 mL, in 50 mM EDTA/PBS diluted (1:100), (Amersham, Cat. No.: 17-5113-01)Compounds: 10 mM in 100% dimethylsulfoxide (DMSO), final conc.: compound 0.003-100 uM in 10% DMSOEnzyme: SYK RPA purified, truncated construct of Spleen Tyrosine Kinase aa 360-635, stock solution 1 mg/mL, MW: 31.2 KDa, final conc.:0.0005 μM.Peptide 1: biotinylated peptide is derived from a naturally occurring phosphor-acceptor consensus sequence (Biotin-EPEGDYEEVLE), special order from QCB, stock solution 20 mM, final conc.: 5.0 μM.ATP: Adenosine-5′-triphosphate 20 mM, (ROCHE Cat. No.: 93202720), final concentration: 20 μMBuffer: HEPES: 2-Hydroxyethyl piperazine-2-ethanesulfonic acid (Sigma, Cat. No.: H-3375)final concentration: 50 mM HEPES pH7.5BSA: Bovine Serum Albumin Fraction V, fatty acid free (Roche Diagnostics GmbH, Cat. No. 9100221) diluted to a final concentration of 0.1%EDTA: EDTA stock solution 500 mM, (GIBCO, Cat. No.: 15575-038) final concentration: 0.1 mMDTT: 1,4-Dithiothreitol (Roche Diagnostics GmbH, Cat. No.: 197777), final conc.: 1 mMMgCl2×6H2O: MERCK, Cat. No.: 105833.1000, final concentration: 10 mMAssay Dilution Buffer (ADB): 50 mM HEPES, 0.1 mM EGTA, 0.1 mM Na Vanadate, 0.1 mM β-glycerophosphate, 10 mM MgCl2, 1 mM DTT, 0.1% BSA, pH 7.5Bead wash buffer: 10 g/L PBS (Phosphate buffered saline) with 2M NaCl+1% phosphoric acid.
- WT-EGFR Kinase Assay For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μL of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine-tri-phosphate (ATP, 3.33 mM=>final conc. in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 7.6 pg/μL. The reaction was stopped by the addition of 3 μL of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, a terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66-Tb-cryptate PT66-Eu-Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5). The resulting mixture was incubated 1 h at 22° C. to allow the binding of the biotinylated phosphorylated peptide to the streptavidine-XL665 and the PT66-Tb-Cryptate.
- Bub1 High ATP Kinase Assay For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 3 μl of a solution of adenosine-tri-phosphate (ATP, 3.33 mM=>final conc. in the 5 μl assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added. Then the kinase reaction was started by the addition of 2 μl of a solution of Bub1 in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Bub1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, a typical concentration is about 200 ng/ml. The reaction was stopped by the addition of 3 μl of a solution of TR-FRET detection reagents (0.167 μM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-002] and 0.67 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, as an alternative a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]) in an aqueous EDTA-solution (83.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES pH 7.5). The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Eu-chelate to the streptavidine-XL.
- CDK2/CycE High ATP Kinase Assay Recombinant fusion proteins of GST and human CDK2 and of GST and human CycE, expressed in insect cells (Sf9) and purified by Glutathion-Sepharose affinity chromatography, were purchase from ProQinase GmbH (Freiburg, Germany). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI peptide technologies (Berlin, Germany). For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of CDK2/CycE in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution ATP (3.33 mM=>final conc. in the 5 μl assay volume is 2 mM) and substrate (1.25 μM=>final conc. in the 5 μl assay volume is 0.75 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK2/CycE was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 15 ng/ml. The reaction was stopped by the addition of 5 μl of a solution of TR-FRET detection reagents (0.2 μM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, as an alternative a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0).
- CDK2/CycE Kinase Assay Method 2: Recombinant fusion proteins of GST and human CDK2 and of GST and human CycE, expressed in insect cells (Sf9) and purified by Glutathion-Sepharose affinity chromatography, were purchased from ProQinase GmbH (Freiburg, Germany). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI Peptide Technologies (Berlin, Germany).For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK2/CycE in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and substrate (1.25 uM=>final conc. in the 5 ul assay volume is 0.75 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK2/CycE was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 130 ng/ml. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0).
- CDK2/CycE Kinase Assay Recombinant fusion proteins of GST and human CDK2 and of GST and human CycE, expressed in insect cells (Sf9) and purified by Glutathion-Sepharose affinity chromatography, were purchased from ProQinase GmbH (Freiburg, Germany). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI Peptide Technologies (Berlin, Germany). For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of CDK2/CycE in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μl assay volume is 10 μM) and substrate (1.25 μM=>final conc. in the 5 μl assay volume is 0.75 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK2/CycE was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 130 ng/mL. The reaction was stopped by the addition of 5 μl of a solution of TR-FRET detection reagents (0.2 μM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0).
- CDK9/CycT1 High ATP Kinase Assay Method 1b: Recombinant full-length His-tagged human CDK9 and CycT1, expressed in insect cells and purified by Ni-NTA affinity chromatography, were purchase from Invitrogen (Cat. No PV4131). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI peptide technologies (Berlin, Germany).For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 3.3 mM=>final conc. in the 5 ul assay volume is 2 mM) and substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK9/CycT1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 0.5 ug/ml. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0).
- CDK9/CycT1 Kinase Assay Method 1a: Recombinant full-length His-tagged human CDK9 and CycT1, expressed in insect cells and purified by Ni-NTA affinity chromatography, were purchased from Invitrogen (Cat. No PV4131). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI Peptide Technologies (Berlin, Germany).For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK9/CycT1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 1 ug/ml. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0).
- CDK9/CycT1 kinase assay Recombinant full-length His-tagged human CDK9 and CycT1, expressed in insect cells and purified by Ni-NTA affinity chromatography, were purchased from Invitrogen (Cat. No PV4131). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI Peptide Technologies (Berlin, Germany). For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μl assay volume is 10 μM) and substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK9/CycT1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 1 μg/mL. The reaction was stopped by the addition of 5 μl of a solution of TR-FRET detection reagents (0.2 μM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0).
- JAK3 Activity Inhibitory Assay In the kinase reactions, fused proteins (6His tag-fused hJAK3 kinase domain (aa781-end)) which were coexpressed in Sf21 cells and purified by Ni2+/NTA agarose were used. The kinase reactions were initiated by the addition of the following solutions of (a) to (c) to 96-well half-area white plates (plates, Corning Incorporated 3642).(a) 5 gmol/L TK substrate-biotin (cisbio) diluted by kinase buffer (50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.0)), 0.02% sodium azide, 0.1 mmol/L sodium vanadate, 5 mmol/L magnesium chloride, 1 mmol/L dithiothreitol, 0.01% bovine serum albumin), 25 μmol/L ATP, 250 nmol/L Supplement Enzymatic buffer (cisbio) solution: 10 μL/well(b) Test-article solution prepared by using kinase buffer containing 5% dimethylsulfoxide: 10 μL/well(c) 33 ng/mL hJAK3 enzyme diluted by kinase buffer: 30 μL/wellA well in which ATP was not added was set out as a blank well.Plates were let stand at room temperature for 10 minutes from starting reactions.To the plates were added 50 μL/well of a buffer for detection containing TK-Antibody-Cryptate (5 tests/50 μL) and streptoavidine-addition XL665 (62.5 nmol/L) reagent (50 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.0), 20 mM EDTA, 800 mmol/L potassium fluoride, 0.1% bovine serum albumin).One hour after the addition of the buffer for detection, fluorescence counts of each well were measured by a fluorescence microplate reader. Specifically, fluorescence counts in 620 nm excited in 337 nm, and fluorescence counts in 665 nm excited by fluorescence in 620 nm were measured.Ratio of each well was calculated from measured fluorescence counts (fluorescence counts in 665 nm/fluorescence counts in 620 nm×10000).Data were obtained by deducting the average Ratio of a blank well from Ratio of each well. IC50 values of test articles were calculated from % of control values of 2 doses before as well as after 50% in 100% as % of a control value of a solvent control. % Inhibition of either 0.1 μmol/L or 1 μmol/L (100-% of control values) was also calculated.
- Kinase Assay For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μl assay volume is 10 μM) and peptide substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 μl assay volume). The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, Perkin Elmer LAS, Rodgau-Jügesheinn, Germany]. The resulting mixture was incubated 1 h at 22° C. to allow the binding of the phosphorylated peptide to the anti-phospho(Ser/Thr)-Europium-antibody. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Europium-labelled anti-phospho(Ser/Thr) antibody to the Streptavidin-XLent. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a Viewlux TR-FRET reader (PerkinElmer LAS, Rodgau-Jügesheinn, Germany).
- Mps-1 Kinase Assay For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Mps-1 in assay buffer [0.1 mM sodium- ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 uM=> final conc. in the 5 ul assay volume is 10 uM) and peptide substrate (1.67 uM=> final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 ul assay volume). The reaction was stopped by the addition of 3 ul of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-Jugesheim, Germany]. The resulting mixture was incubated 1 h at 22° C. to allow the binding of the phosphorylated peptide to the anti-phospho(Ser/Thr)-Europium-antibody. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Europium-labelled anti-phospho(Ser/Thr) antibody to the Streptavidin-XLent. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a Viewlux TR-FRET reader (PerkinElmer LAS, Rodgau-Jugesheim, Germany).
- Selective Inhibition Assays of Isolated Na,K-ATPase To screen for isoform selectivity of the digoxin derivatives we compared inhibition of Na,K-ATPase activity of purified detergent-soluble human isoform complexes α1β1FXYD1, α2β1FXYD1, α2β2FXTD1 and α2β3FXYD1. Although all the preparations and assays were conducted with FXYD1 in order to stabilize the complexes, the FXYD1 suffix is omitted in naming of isoform complexes for simplicity.Na,K-ATPase activity of α/βPFXYD1 complexes was measured over one hour at 37° C. in a medium containing 130 mM NaCl, 5 mM KCl, 3 mM MgCl2, 1 mM EGTA, 25 mM Histidine, pH 7.4 and 1 mM ATP using the PiColor Lock gold malachite green assay (Inova Biosciences).The Na,K-ATPase activities were α1β1, 21.5±5.3 μmoles/min/mg; α2β1, 18.7±1.8 μmoles/min/mg, and α2β3, 10.7±1.9 μmoles/min/mg protein. As discussed below, an important kinetic property in relation to inhibition by cardiac glycosides is K0.5 for activation by K: α1β1-1.25±0.05 mM, α2β1-2.7±0.14 mM and α2β3 6.4±0.50 mM, respectively.Selectivity of the compounds for various isolated isoforms of human Na,K-ATPase was determined essentially as described before [Katz, A. et al., J Biol Chem., 2010, 285(25), pp. 19582-19592].ATPase activity assays as well as titrations with NaCl, KCl and vanadate were performed as described in Lifshitz-2007 and Loayza-1998 using PiColorLock malachite green assay (Inova Bioscience). Inhibitor assays were performed as described in Katz-2010. [3H]ouabain binding and K+-[3H]digoxin displacement assays were performed as described in Katz-2010.The percent inhibition VCG/V0 was calculated and Ki values were obtained by fitting the data to the function VCG/V0=Ki/([CG]+Ki)+c (CG stands for cardiac glycoside). Inhibition was estimated in 3-5 separate experiments and average Ki values±standard error of the mean (SEM) were calculated. The ratios Ki α1β1/α2β1, α1β1/α2β2 and α1β1/α2β3 was calculated for each compound.
- Time-Resolved Fluorescence Energy Transfer (TR-FRET) Kinase Assay In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μM, 5.9 μM and 20 μM) were tested in duplicate within the same microtiter plate. To this end, 100-fold concentrated compound solutions (in DMSO) were previously prepared by serial dilution (1:3.4) of 2 mM stocks in a clear low volume 384-well source microtiter plate (Greiner Bio-One, Frickenhausen, Germany), from which 50 nL of compounds were transferred into a black low volume test microtiter plate from the same supplier. Subsequently, 2 μL of Bub1 (the final concentration of Bub1 was adjusted depending on the activity of the enzyme lot in order to be within the linear dynamic range of the assay: typically 200 ng/mL were used) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added to the compounds in the test plate and the mixture was incubated for 15 min at 22° C. to allow pre-equilibration of the putative enzyme-inhibitor complexes before the start of the kinase reaction, which was initiated by the addition of 3 μL 1.67-fold concentrated solution (in assay buffer) of adenosine-tri-phosphate (ATP, 10 μM final concentration) and peptide substrate (1 μM final concentration). The resulting mixture (5 μL final volume) was incubated at 22° C. during 60 min., and the reaction was stopped by the addition of 5 μL of an aqueous EDTA-solution (50 mM EDTA, in 100 mM HEPES pH 7.5 and 0.2% (w/v) bovine serum albumin) which also contained the TR-FRET detection reagents (0.2 μM streptavidin-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-001] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no.
- WT-EGFR Kinase Assay For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μL of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine-tri-phosphate (ATP, 3.33 mM=>final conc. in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 7.6 pg/μL. The reaction was stopped by the addition of 3 μL of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, a terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66-Tb-cryptate PT66-Eu-Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5).The resulting mixture was incubated 1 h at 22° C. to allow the binding of the biotinylated phosphorylated peptide to the streptavidine-XL665 and the PT66-Tb-Cryptate. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the PT66-Tb-Cryptate to the streptavidine-XL665.
- Bub1 Kinase Assay Recombinant fusion proteins of GST and human CDK2 and of GST and human CycE, expressed in insect cells (Sf9) and purified by Glutathion-Sepharose affinity chromatography, were purchased from ProQinase GmbH (Freiburg, Germany). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI peptide technologies (Berlin, Germany). For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK2/CycE in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and substrate (1.25 uM=>final conc. in the 5 ul assay volume is 0.75 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK2/CycE was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 130 ng/ml. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, as an alternative a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0). The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents.
- CDK2/CycE Kinase Assay For the assay 50 nanoL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 microL of a solution of CDK2/CycE in aqueous assay buffer [50 millimol/L Tris/HCl PH 8.0, 10 millimol/L MgCl2, 1.0 millimol/L dithiothreitol, 0.1 millimol/L sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 microL of a solution of adenosine-tri-phosphate (ATP, 3.33 millimol/L=>final conc. in the 5 microL assay volume is 2 millimol/L) and substrate (1.25 micromol/L=>final conc. in the 5 microL assay volume is 0.75 micromol/L) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK2/CycE was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 10 ng/ml. The reaction was stopped by the addition of 3 microL of a solution of TR-FRET detection reagents (0.333 micromol/L streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nanomol/L anti-RB (pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 2 nanomol/L LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, as an alternative a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]) in an aqueous EDTA-solution (167 millimol/L EDTA, 0.2% (w/v) bovine serum albumin in 100 millimol/L HEPES pH 7.5).The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Eu-chelate to the streptavidine-XL. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a TR-FRET reader, e.g. a Pherastar (BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer).
- CDK9/CycT1 High ATP Kinase Assay For the assay 50 nanoL of a 100 fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 microL of a solution of CDK9/CycT1 in aqueous assay buffer [50 millimol/L Tris/HCl PH 8.0, 10 millimol/L MgCl2, 1.0 millimol/L dithiothreitol, 0.1 millimol/L sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 microL of a solution of adenosine-tri-phosphate (ATP, 3.3 millimol/L=>final conc. in the 5 microL assay volume is 2 millimol/L) and substrate (1.25 micromol/L=>final conc. in the 5 microL assay volume is 0.75 micromol/L) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK9/CycT1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 0.5 microg/ml. The reaction was stopped by the addition of 3 microL of a solution of TR-FRET detection reagents (0.33 micromol/L streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nanomol/L anti-RB (pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 2 nanomol/L LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (167 millimol/L EDTA, 0.2% (w/v) bovine serum albumin in 100 millimol/L HEPES pH 7.5).The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Eu-chelate to the streptavidine-XL. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a TR-FRET reader, e.g. a Pherastar (BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer).
- Kinase Assay In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μM, 5.9 μM and 20 μM) were tested in duplicate within the same microtiter plate. To this end, 100-fold concentrated compound solutions (in DMSO) were previously prepared by serial dilution (1:3.4) of 2 mM stocks in a clear low volume 384-well source microtiter plate (Greiner Bio-One, Frickenhausen, Germany), from which 50 nl of compounds were transferred into a black low volume test microtiter plate from the same supplier. Subsequently, 2 μL of Bub1 (the final concentration of Bub1 was adjusted depending on the activity of the enzyme lot in order to be within the linear dynamic range of the assay: typically 200 ng/mL were used) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added to the compounds in the test plate and the mixture was incubated for 15 min at 22° C. to allow pre-equilibration of the putative enzyme-inhibitor complexes before the start of the kinase reaction, which was initiated by the addition of 3 μL 1.67-fold concentrated solution (in assay buffer) of adenosine-tri-phosphate (ATP, 10 μM final concentration) and peptide substrate (1 μM final concentration). The resulting mixture (5 μL final volume) was incubated at 22° C. during 60 min., and the reaction was stopped by the addition of 5 μL of an aqueous EDTA-solution (50 mM EDTA, in 100 mM HEPES pH 7.5 and 0.2% (w/v) bovine serum albumin) which also contained the TR-FRET detection reagents (0.2 μM streptavidin-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-002] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, alternatively a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]). The stopped reaction mixture was further incubated 1 h at 22° C. in order to allow the formation of complexes between peptides and detection reagents.
- Kinase Assay In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μM, 5.9 μM and 20 μM) were tested in duplicate within the same microtiter plate. To this end, 100-fold concentrated compound solutions (in DMSO) were previously prepared by serial dilution (1:3.4) of 2 mM stocks in a clear low volume 384-well source microtiter plate (Greiner Bio-One, Frickenhausen, Germany), from which 50 nl of compounds were transferred into a black low volume test microtiter plate from the same supplier. Subsequently, 2 μl of Bub1 (the final concentration of Bub1 was adjusted depending on the activity of the enzyme lot in order to be within the linear dynamic range of the assay: typically 200 ng/ml were used) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added to the compounds in the test plate and the mixture was incubated for 15 min at 22° C. to allow pre-equilibration of the putative enzyme-inhibitor complexes before the start of the kinase reaction, which was initiated by the addition of 3 μl 1.67-fold concentrated solution (in assay buffer) of adenosine-tri-phosphate (ATP, 10 μM final concentration) and peptide substrate (1 μM final concentration). The resulting mixture (5 μl final volume) was incubated at 22° C. during 60 min., and the reaction was stopped by the addition of 5 μl of an aqueous EDTA-solution (50 mM EDTA, in 100 mM HEPES pH 7.5 and 0.2% (w/v) bovine serum albumin) which also contained the TR-FRET detection reagents (0.2 μM streptavidin-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-002] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, alternatively a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]).
- Mps-1 Kinase Assay N-terminally GST-tagged human full length recombinant Mps-1 kinase (purchased from Invitrogen, Karslruhe, Germany, cat. no PV4071) was used. As substrate for the kinase reaction a biotinylated peptide of the amino-acid sequence biotin-Ahx-PWDPDDADITEILG (C-terminus in amide form, purchased from Biosyntan GmbH, Berlin) was used. For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA (w/v), 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of 16.7 uM adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and peptide substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 0.5 nM (final conc. in the 5 ul assay volume). The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-Jugesheim, Germany]. Instead of the 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody a mixture of 2 nM unlabeled anti-phospho ser/thr-pro antibody MPM-2 [Millipore cat. #05-368] and 1 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077] can be used). The resulting mixture was incubated 1 h at 22° C. to allow the binding of the phosphorylated peptide to the anti-phospho(Ser/Thr)-Europium-antibody.
- Time-Resolved Fluorescence Resonance Energy Transfer (TR-FRET) Assay The human kinase Mps-1 phosphorylates a biotinylated substrate peptide. Detection of the phosphorylated product is achieved by time-resolved fluorescence resonance energy transfer (TR-FRET) from Europium-labelled anti-phospho-Serine/Threonine antibody as donor to streptavidin labelled with cross-linked allophycocyanin (SA-XLent) as acceptor. Compounds are tested for their inhibition of the kinase activity.N-terminally GST-tagged human full length recombinant Mps-1 kinase (purchased from Invitrogen, Karslruhe, Germany, cat. no PV4071) was used. As substrate for the kinase reaction a biotinylated peptide of the amino-acid sequence PWDPDDADITEILG (C-terminus in amide form, purchased from Biosynthan GmbH, Berlin) was used.For the assay 50 nL of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and peptide substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 ul assay volume). The reaction was stopped by the addition of 3 ul of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-J gesheim, Germany].
- WT-EGFR Kinase Assay Recombinant fusion protein of N-terminal Glutathion-S-Transferase (GST) and a fragment of human EGFR (amino acids R669 to A1210), expressed in Sf9 insect cells and purified via affinity chromatography using Glutathion Sepharose as described above, was used as kinase. As substrate for the kinase reaction the biotinylated peptide biotin-Ahx-AEEEEYFELVAKKK (C-terminus in amide form) was used which can be purchased e.g. form the company Biosynthan GmbH (Berlin-Buch, Germany).For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μL of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine-tri-phosphate (ATP, 3.33 mM=>final conc. in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 7.6 pg/μL. The reaction was stopped by the addition of 3 μL of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, a terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66-Tb-cryptate PT66-Eu-Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5).The resulting mixture was incubated 1 h at 22° C. to allow the binding of the biotinylated phosphorylated peptide to the streptavidine-XL665 and the PT66-Tb-Cryptate.
- CDK2/CycE Kinase Assay N-terminally His6-tagged recombinant catalytic domain of human Bub1 (amino acids 704-1085), expressed in insect cells (Hi5) and purified by Ni-NTA affinity chromatography and subsequent size exclusion chromatography, was used as enzyme. As substrate for the kinase reaction the biotinylated peptide biotin-Ahx-VLLPKKSFAEPG (C-terminus in amid form) was used which can be purchased e.g. form the company Biosyntan (Berlin, Germany). For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of Bub1 in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1x Complete EDTA-free protease inhibitor mixture (Roche)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 16.7 uM=>final conc. in the 5 ul assay volume is 10 uM) and substrate (1.67 uM=>final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Bub1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 200 ng/ml. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-001] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, as an alternative a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]) in an aqueous EDTA-solution (50 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES pH 7.5). The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents.
- Mps-1 Kinase Assay For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μl assay volume is 10 μM) and peptide substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 μl assay volume). The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-J gesheim, Germany].The resulting mixture was incubated 1 h at 22° C. to allow the binding of the phosphorylated peptide to the anti-phospho(Ser/Thr)-Europium-antibody. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Europium-labelled anti-phospho(Ser/Thr) antibody to the Streptavidin-XLent. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a Viewlux TR-FRET reader (PerkinElmer LAS, Rodgau-J gesheim, Germany). The “blank-corrected normalized ratio” (a Viewlux specific readout, similar to the traditional ratio of the emissions at 665 nm and at 622 nm, in which blank and Eu-donor crosstalk are subtracted from the 665 nm signal before the ratio is calculated) was taken as the measure for the amount of phosphorylated substrate. The data were normalised (enzyme reaction without inhibitor=0% inhibition, all other assay components but no enzyme=100% inhibition).
- Exon20-Mutant-EGFR(V769_D770insASV) Kinase Assay A recombinant fusion protein of N-terminal Glutathion-S-Transferase (GST) and a fragment of human EGFR variant (amino acids R669 to A1210 with insertion of the amino acids sequence ASV between V769 and D770; ( EGFR ins ASV ), expressed in Sf9 insect cells and purified via affinity chromatography using Glutathion Sepharose as described above, was used as kinase. As substrate for the kinase reaction the biotinylated peptide biotin-Ahx-AEEEEYFELVAKKK SEQ ID 6 (C-terminus in amide form) was used which can be purchased e.g. form the company Biosynthan GmbH (Berlin-Buch, Germany). For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22° C. to allow pre binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μL of a solution of adenosine tri phosphate (ATP, 3.33 mM=>final conc. in the 5 μL assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μL assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22° C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 2.5 μg/μl. The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, an terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66 Tb cryptate PT66 Eu Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5). The resulting mixture was incubated 1 h at 22° C. to allow the binding of the biotinylated phosphorylated peptide to the streptavidine-XL665 and the PT66-Tb-Cryptate. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the PT66-Tb-Cryptate to the streptavidine-XL665.
- CDK2/CycE Kinase Assay For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK2/CycE in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 16.7 uM=> final conc. in the 5 ul assay volume is 10 uM) and substrate (1.25 uM=> final conc. in the 5 ul assay volume is 0.75 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK2/CycE was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 130 ng/mL. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0). The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Eu-chelate to the streptavidine-XL. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a TR-FRET reader, e.g. a Rubystar (BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer). The ratio of the emissions at 665 nm and at 622 nm was taken as the measure for the amount of phosphorylated substrate. The data were normalised (enzyme reaction without inhibitor=0% inhibition, all other assay components but no enzyme=100% inhibition). Usually the test compounds were tested on the same microtiterplate in 11 different concentrations in the range of 20 uM to 0.1 nM (20 uM, 5.9 uM, 1.7 uM, 0.51 uM, 0.15 uM, 44 nM, 13 nM, 3.8 nM, 1.1 nM, 0.33 nM and 0.1 nM, the dilution series prepared separately before the assay on the level of the 100 fold concentrated solutions in DMSO by serial 1:3.4 dilutions) in duplicate values for each concentration and IC50 values were calculated by a 4 parameter fit.
- CDK9/CycT1 Kinase Assay For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 ul of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 ul of a solution of adenosine-tri-phosphate (ATP, 16.7 uM=> final conc. in the 5 ul assay volume is 10 uM) and substrate (1.67 uM=> final conc. in the 5 ul assay volume is 1 uM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK9/CycT1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 1 ug/mL. The reaction was stopped by the addition of 5 ul of a solution of TR-FRET detection reagents (0.2 uM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0). The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Eu-chelate to the streptavidine-XL. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a HTRF reader, e.g. a Rubystar (BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer). The ratio of the emissions at 665 nm and at 622 nm was taken as the measure for the amount of phosphorylated substrate. The data were normalised (enzyme reaction without inhibitor=0% inhibition, all other assay components but no enzyme=100% inhibition). Usually the test compounds were tested on the same microtiterplate in 11 different concentrations in the range of 20 uM to 0.1 nM (20 uM, 5.9 uM, 1.7 uM, 0.51 uM, 0.15 uM, 44 nM, 13 nM, 3.8 nM, 1.1 nM, 0.33 nM and 0.1 nM, the dilution series prepared separately before the assay on the level of the 100 fold concentrated solutions in DMSO by serial 1:3.4 dilutions) in duplicate values for each concentration and IC50 values were calculated by a 4 parameter fit.
- Mps-1 Kinase Assay For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of Mps-1 in assay buffer [0.1 mM sodium-ortho-vanadate, 10 mM MgCl2, 2 mM DTT, 25 mM Hepes pH 7.7, 0.05% BSA, 0.001% Pluronic F-127] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to Mps-1 before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of 16.7 adenosine-tri-phosphate (ATP, 16.7 μM=>final conc. in the 5 μl assay volume is 10 μM) and peptide substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 60 min at 22° C. The concentration of Mps-1 in the assay was adjusted to the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical enzyme concentrations were in the range of about 1 nM (final conc. in the 5 μl assay volume). The reaction was stopped by the addition of 3 μl of a solution of HTRF detection reagents (100 mM Hepes pH 7.4, 0.1% BSA, 40 mM EDTA, 140 nM Streptavidin-XLent [#61GSTXLB, Fa. Cis Biointernational, Marcoule, France], 1.5 nM anti-phospho(Ser/Thr)-Europium-antibody [#AD0180, PerkinElmer LAS, Rodgau-J gesheinn, Germany]. The resulting mixture was incubated 1 h at 22° C. to allow the binding of the phosphorylated peptide to the anti-phospho(Ser/Thr)-Europium-antibody. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Europium-labelled anti-phospho(Ser/Thr) antibody to the Streptavidin-XLent. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a Viewlux TR-FRET reader (PerkinElmer LAS, Rodgau-J gesheinn, Germany). The blank-corrected normalized ratio (a Viewlux specific readout, similar to the traditional ratio of the emissions at 665 nm and at 622 nm, in which blank and Eu-donor crosstalk are subtracted from the 665 nm signal before the ratio is calculated) was taken as the measure for the amount of phosphorylated substrate. The data were normalised (enzyme reaction without inhibitor=0% inhibition, all other assay components but no enzyme=100% inhibition). Test compounds were tested on the same microtiter plate at 10 different concentrations in the range of 20 μM to 1 nM (20 μM, 6.7 μM, 2.2 μM, 0.74 μM, 0.25 μM, 82 nM, 27 nM, 9.2 nM, 3.1 nM and 1 nM, dilution series prepared before the assay at the level of the 100 fold conc. stock solutions by serial 1:3 dilutions) in duplicate values for each concentration and IC50 values were calculated by a 4 parameter fit using an inhouse software.
- CDK9/CycT1 High ATP Kinase Assay Recombinant full-length His-tagged human CDK9 and CycT1, expressed in insect cells and purified by Ni-NTA affinity chromatography, were purchase from Invitrogen (Cat. No PV4131). As substrate for the kinase reaction biotinylated peptide biotin-Ttds-YISPLKSPYKISEG (C-terminus in amid form) was used which can be purchased e.g. form the company JERINI peptide technologies (Berlin, Germany).For the assay 50 nl of a 100 fold concentrated solution of the test compound in DMSO was pipetted into a black low volume 384 well microtiter plate (Greiner Bio-One, Frickenhausen, Germany), 2 μl of a solution of CDK9/CycT1 in aqueous assay buffer [50 mM Tris/HCl pH 8.0, 10 mM MgCl2, 1.0 mM dithiothreitol, 0.1 mM sodium ortho-vanadate, 0.01% (v/v) Nonidet-P40 (Sigma)] were added and the mixture was incubated for 15 min at 22° C. to allow pre-binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 μl of a solution of adenosine-tri-phosphate (ATP, 3.3 mM=>final conc. in the 5 μl assay volume is 2 mM) and substrate (1.67 μM=>final conc. in the 5 μl assay volume is 1 μM) in assay buffer and the resulting mixture was incubated for a reaction time of 25 min at 22° C. The concentration of CDK9/CycT1 was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentrations were in the range of 0.5 μg/mL. The reaction was stopped by the addition of 5 μl of a solution of TR-FRET detection reagents (0.2 μM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-RB(pSer807/pSer811)-antibody from BD Pharmingen [#558389] and 1.2 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077]) in an aqueous EDTA-solution (100 mM EDTA, 0.2% (w/v) bovine serum albumin in 100 mM HEPES/NaOH pH 7.0). The resulting mixture was incubated 1 h at 22° C. to allow the formation of complex between the phosphorylated biotinylated peptide and the detection reagents. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the Eu-chelate to the streptavidine-XL. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 350 nm was measured in a HTRF reader, e.g. a Rubystar (BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer). The ratio of the emissions at 665 nm and at 622 nm was taken as the measure for the amount of phosphorylated substrate. The data were normalised (enzyme reaction without inhibitor=0% inhibition, all other assay components but no enzyme=100% inhibition). Usually the test compounds were tested on the same microtiterplate in 11 different concentrations in the range of 20 μM to 0.1 nM (20 μM, 5.9 μM, 1.7 μM, 0.51 μM, 0.15 μM, 44 nM, 13 nM, 3.8 nM, 1.1 nM, 0.33 nM and 0.1 nM, the dilution series prepared separately before the assay on the level of the 100 fold concentrated solutions in DMSO by serial 1:3.4 dilutions) in duplicate values for each concentration and IC50 values were calculated by a 4 parameter fit using an inhouse software.
- Bub1 Kinase Assay Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRET) kinase assay which measures phosphorylation of the synthetic peptide Biotin-Ahx-VLLPKKSFAEPG (SEQ ID No. 1) (C-terminus in amide form), purchased from e.g. Biosyntan (Berlin, Germany) by the (recombinant) catalytic domain of human Bub1 (amino acids 704-1085), expressed in Hi5 insect cells with an N-terminal His6-tag and purified by affinity-(Ni-NTA) and size exclusion chromatography.In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μM, 5.9 μM and 20 μM) were tested in duplicate within the same microtiter plate. To this end, 100-fold concentrated compound solutions (in DMSO) were previously prepared by serial dilution (1:3.4) of 2 mM stocks in a clear low volume 384-well source microtiter plate (Greiner Bio-One, Frickenhausen, Germany), from which 50 nl of compounds were transferred into a black low volume test microtiter plate from the same supplier. Subsequently, 2 μL of Bub1 (the final concentration of Bub1 was adjusted depending on the activity of the enzyme lot in order to be within the linear dynamic range of the assay: typically 200 ng/mL were used) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added to the compounds in the test plate and the mixture was incubated for 15 min at 22° C. to allow pre-equilibration of the putative enzyme-inhibitor complexes before the start of the kinase reaction, which was initiated by the addition of 3 μL 1.67-fold concentrated solution (in assay buffer) of adenosine-tri-phosphate (ATP, 10 μM final concentration) and peptide substrate (1 μM final concentration). The resulting mixture (5 μL final volume) was incubated at 22° C. during 60 min. and the reaction w as stopped by the addition of 5 μL of an aqueous EDTA-solution (50 mM EDTA, in 100 mM HEPES pH 7.5 and 0.2% (w/v) bovine serum albumin) which also contained the TR-FRET detection reagents (0.2 μM streptavidin-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-002] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, alternatively a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]). The stopped reaction mixture was further incubated 1 h at 22° C. in order to allow the formation of complexes between peptides and detection reagents. Subsequently, the amount of product was evaluated by measurement of the resonance energy transfer from the Eu-chelate-antibody complex recognizing the Phosphoserine residue to the streptavidin-XL665 bound to the biotin moiety of the peptide. To this end, the fluorescence emissions at 620 nm and 665 nm after excitation at 330-350 nm were measured in a TR-FRET plate reader, e.g. a Rubystar or Pherastar (both from BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer) and the ratio of the emissions (665 nm/622 nm) was taken as indicator for the amount of phosphorylated substrate. The data were normalised using two sets of control wells for high−(=enzyme reaction without inhibitor=0%=Minimum inhibition) and low−(=all assay components without enzyme=100%=Maximum inhibition) Bub1 activity. IC50 values were calculated by fitting the normalized inhibition data to a 4-parameter logistic equation (Minimum, Maximum, IC50, Hill; Y=Max+(Min−Max)/(1+(X/IC50)Hill)).
- Bub1 Kinase Assay Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRET) kinase assay which measures phosphorylation of the synthetic peptide Blotin-AhxVLLPKKSFAEPG (C-terminus in amide form), purchased from e.g. Biosyntan (Berlin, Germany) by the (recombinant) catalytic domain of human Bub1 (amino acids 704-1085), expressed in HI5 insect cells with an N-terminal HIs6-tag and purified by affinity-(Ni-NTA) and size exclusion chromatography.In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μM, 5.9 μM and 20 μM) were tested in duplicate within the same microtiter plate. To this end, 100-fold concentrated compound solutions (in DMSO) were previously prepared by serial dilution (1:3.4) of 2 mM stocks in a clear low volume 384-well source microtiter plate (Greiner Bio-One, Frickenhausen, Germany), from which 50 nl of compounds were transferred into a black low volume test microtiter plate from the same supplier. Subsequently, 2 μl of Bub1 (the final concentration of Bub1 was adjusted depending on the activity of the enzyme lot in order to be within the linear dynamic range of the assay: typically 200 μg/ml were used) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCl), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added to the compounds in the test plate and the mixture was incubated for 15 min at 229 to allow pre-equilibration of the putative enzyme-inhibitor complexes before the start of the kinase reaction, which was initiated by the addition of 3 μl 1.67-fold concentrated solution (in assay buffer) of adenosine-tri-phosphate (ATP, 10 μM final concentration) and peptide substrate (1 μM final concentration). The resulting mixture (5 μl final volume) was incubated at 22° C. during 60 min., and the reaction was stopped by the addition of 5 μl of an aqueous EDTA-solution (50 mM EDTA, in 100 mM HEPES pH 7.5 and 0.2% (w/v) bovine serum albumin) which also contained the TR-FRET detection reagents (0.2 μM streptavidin-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-001] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, alternatively a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]). The stopped reaction mixture was further incubated 1 h at 22° C. in order to allow the formation of complexes between peptides and detection reagents. Subsequently, the amount of product was evaluated by measurement of the resonance energy transfer from the Eu-chelate-antlbody complex recognizing the Phosphoserlne residue to the streptavidin-XL665 bound to the biotin moiety of the peptide. To this end, the fluorescence emissions at 620 nm and 665 nm after excitation at 330-350 nm were measured in a TR-FRET plate reader, e.g. a Rubystar or Pherastar (both from BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer) and the ratio of the emissions (665 nm/622 nm) was taken as indicator for the amount of phosphorylated substrate. The data were normalised using two sets of (typically 32-) control wells for high-(=enzyme reaction without Inhibitor=0%=Minimum inhibition) and low-(=all assay components without enzyme=100%=Maximum inhibition) Bub1 activity. IC50 values were calculated by fitting the normalized inhibition data to a 4-parameter logistic equation (Minimum, Maximum, IC50, Hill; Y=Max+(Min−Max)/(1+(X/IC50)Hill)) using a Bayer-proprietary analysis software.
- Bub1 kinase assay Bub1-inhibitory activities of compounds described in the present invention were quantified using a time-resolved fluorescence energy transfer (TR-FRET) kinase assay which measures phosphorylation of the synthetic peptide Biotin-Ahx-VLLPKKSFAEPG (SEQ ID No.1) (C-terminus in amide form), purchased from e.g. Biosyntan (Berlin, Germany) by the (recombinant) catalytic domain of human Bub1 (amino acids 704-1085), expressed in Hi5 insect cells with an N-terminal His6-tag and purified by affinity-(Ni-NTA) and size exclusion chromatography.In a typical assay 11 different concentrations of each compound (0.1 nM, 0.33 nM, 1.1 nM, 3.8 nM, 13 nM, 44 nM, 0.15 μM, 0.51 μM, 1.7 μM, 5.9 μM and 20 μM) were tested in duplicate within the same microtiter plate. To this end, 100-fold concentrated compound solutions (in DMSO) were previously prepared by serial dilution (1:3.4) of 2 mM stocks in a clear low volume 384-well source microtiter plate (Greiner Bio-One, Frickenhausen, Germany), from which 50 nl of compounds were transferred into a black low volume test microtiter plate from the same supplier. Subsequently, 2 μL of Bub1 (the final concentration of Bub1 was adjusted depending on the activity of the enzyme lot in order to be within the linear dynamic range of the assay: typically 200 ng/mL were used) in aqueous assay buffer [50 mM Tris/HCl pH 7.5, 10 mM magnesium chloride (MgCl2), 200 mM potassium chloride (KCL), 1.0 mM dithiothreitol (DTT), 0.1 mM sodium ortho-vanadate, 1% (v/v) glycerol, 0.01% (w/v) bovine serum albumine (BSA), 0.005% (v/v) Trition X-100 (Sigma), 1× Complete EDTA-free protease inhibitor mixture (Roche)] were added to the compounds in the test plate and the mixture was incubated for 15 min at 22° C. to allow pre-equilibration of the putative enzyme-inhibitor complexes before the start of the kinase reaction, which was initiated by the addition of 3 μL 1.67-fold concentrated solution (in assay buffer) of adenosine-tri-phosphate (ATP, 10 μM final concentration) and peptide substrate (1 μM final concentration). The resulting mixture (5 μL final volume) was incubated at 22° C. during 60 min. and the reaction was stopped by the addition of 5 μL of an aqueous EDTA-solution (50 mM EDTA, in 100 mM HEPES pH 7.5 and 0.2% (w/v) bovine serum albumin) which also contained the TR-FRET detection reagents (0.2 μM streptavidin-XL665 [Cisbio Bioassays, Codolet, France] and 1 nM anti-phosho-Serine antibody [Merck Millipore, cat. #35-002] and 0.4 nM LANCE EU-W1024 labeled anti-mouse IgG antibody [Perkin-Elmer, product no. AD0077, alternatively a Terbium-cryptate-labeled anti-mouse IgG antibody from Cisbio Bioassays can be used]). The stopped reaction mixture was further incubated 1 h at 22° C. in order to allow the formation of complexes between peptides and detection reagents. Subsequently, the amount of product was evaluated by measurement of the resonance energy transfer from the Eu-chelate-antibody complex recognizing the Phosphoserine residue to the streptavidin-XL 665 bound to the biotin moiety of the peptide. To this end, the fluorescence emissions at 620 nm and 665 nm after excitation at 330-350 nm were measured in a TR-FRET plate reader, e.g. a Rubystar or Pherastar (both from BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer) and the ratio of the emissions (665 nm/622 nm) was taken as indicator for the amount of phosphorylated substrate. The data were normalised using two sets of control wells for high (=enzyme reaction without inhibitor=0%=Minimum inhibition) and low (=all assay components without enzyme=100%=Maximum inhibition) Bub1 activity. IC50 values were calculated by fitting the normalized inhibition data to a 4-parameter logistic equation (Minimum, Maximum, IC50, Hill; Y=Max+(Min−Max)/(1+(X/IC50)Hill)).
- Exon20-mutant-EGFR(D770_N771insSVD) kinase assay Inhibitory activity of compounds of the present invention against an Epidermal Growth Factor Receptor (EGFR) with an insertion of the amino acids sequence SVD between D770and N771 was quantified employing the TR-FRET based kinase activity assay as described in the following paragraphs.A recombinant fusion protein of N-terminal Glutathion-S-Transferase (GST) and a fragment of human EGFR variant (amino acids R669 to A1210 with insertion of the amino acids sequence SVD between D770 and N771 ( EGFR ins SVD ), expressed in Sf9 insect cells and purified via affinity chromatography using Glutathion Sepharose as described above, was used as a kinase. As substrate for the kinase reaction the biotinylated peptide biotin-Ahx-AEEEEYFELVAKKK (C-terminus in amide form) was used which can be purchased e.g. form the company Biosynthan GmbH (Berlin-Buch, Germany).For the assay 50 nl of a 100-fold concentrated solution of the test compound in DMSO was pipetted into either a black low volume 384 well microtiter plate or a black 1536 well microtiter plate (both Greiner Bio-One, Frickenhausen, Germany), 2 µl of a solution of EGFR in aqueous assay buffer [50 mM Hepes pH 7.0, 10 mM MgCl2, 1 mM dithiothreitol, 0.5 mM EGTA, 0.3 mM activated sodium ortho-vanadate, 0.005% (w/v) bovine serum albumin, 0.005% (v/v) Tween-20] were added and the mixture was incubated for 15 min at 22°C to allow pre binding of the test compounds to the enzyme before the start of the kinase reaction. Then the kinase reaction was started by the addition of 3 µL of a solution of adenosine tri phosphate (ATP, 3.33 mM => final conc. in the 5 µL assay volume is 2 mM) and substrate (1.67 µM => final conc. in the 5 µL assay volume is 1 µM) in assay buffer and the resulting mixture was incubated for a reaction time of 30 min at 22°C. The concentration of EGFR was adjusted depending of the activity of the enzyme lot and was chosen appropriate to have the assay in the linear range, typical concentration was 15 pg/µl. The reaction was stopped by the addition of 3 µl of a solution of HTRF detection reagents (83.3 nM streptavidine-XL665 [Cisbio Bioassays, Codolet, France] and 1.67 nM PT66-Tb-Cryptate, a terbium-cryptate labelled anti-phospho-tyrosine antibody from Cisbio Bioassays [instead of the PT66 Tb cryptate PT66 Eu Chelate from Perkin Elmer can also be used]) in an aqueous EDTA-solution (133.3 mM EDTA, 0.2% (w/v) bovine serum albumin in 50 mM HEPES pH 7.5). The resulting mixture was incubated 1 h at 22°C to allow the binding of the biotinylated phosphorylated peptide to the streptavidine-XL665 and the PT66-Tb-Cryptate. Subsequently the amount of phosphorylated substrate was evaluated by measurement of the resonance energy transfer from the PT66-Tb-Cryptate to the streptavidine-XL665. Therefore, the fluorescence emissions at 620 nm and 665 nm after excitation at 337 nm were measured in a HTRF reader, e.g. a Pherastar (BMG Labtechnologies, Offenburg, Germany) or a Viewlux (Perkin-Elmer). The ratio of the emissions at 665 nm and at 622 nm was taken as the measure for the amount of phosphorylated substrate. The data were normalised (enzyme reaction without inhibitor = 0% inhibition, all other assay components but no enzyme = 100% inhibition). Usually the test compounds were tested on the same microtiterplate in 11 different concentrations in the range of 20 µM to 0.07 nM (20 µM, 5.7 µM, 1.6 µM, 0.47 µM, 0.13 µM, 38 nM, 11 nM, 3.1 nM, 0.9 nM, 0.25 nM and 0.07 nM, the dilution series prepared separately before the assay on the level of the 100fold concentrated solutions in DMSO by serial dilutions, exact concentrations may vary depending pipettors used) in duplicate values for each concentration and IC50 values were calculated using Genedata Screener software.

