Compound (1)
Article Title (10)
Assay (5)
Ota, Y; Itoh, Y; Kurohara, T; Singh, R; Elboray, EE; Hu, C; Zamani, F; Mukherjee, A; Takada, Y; Yamashita, Y; Morita, M; Horinaka, M; Sowa, Y; Masuda, M; Sakai, T; Suzuki, T Cancer-Cell-Selective Targeting by Arylcyclopropylamine-Vorinostat Conjugates. ACS Med Chem Lett 13: 1568 -1573 (2022) Geurs, S; Clarisse, D; De Bosscher, K; D'hooghe, M The Zinc-Binding Group Effect: Lessons from Non-Hydroxamic Acid Vorinostat Analogs. J Med Chem 66: 7698 -7729 (2023) Hattori, K; Kohchi, Y; Oikawa, N; Suda, H; Ura, M; Ishikawa, T; Miwa, M; Endoh, M; Eda, H; Tanimura, H; Kawashima, A; Horii, I; Ishitsuka, H; Shimma, N Design and synthesis of the tumor-activated prodrug of dihydropyrimidine dehydrogenase (DPD) inhibitor, RO0094889 for combination therapy with capecitabine. Bioorg Med Chem Lett 13: 867 -72 (2003) Huang, M; Xie, X; Gong, P; Wei, Y; Du, H; Xu, Y; Xu, Q; Jing, Y; Zhao, L A 18β-glycyrrhetinic acid conjugate with Vorinostat degrades HDAC3 and HDAC6 with improved antitumor effects. Eur J Med Chem 188: (2020) Erdeljac, N; Bussmann, K; Schöler, A; Hansen, FK; Gilmour, R Fluorinated Analogues of the Histone Deacetylase Inhibitor Vorinostat (Zolinza): Validation of a Chiral Hybrid Bioisostere, BITE. ACS Med Chem Lett 10: 1336 -1340 (2019) Yao, L; Ramanujulu, PM; Poulsen, A; Ohlson, S; Dymock, BW Merging of ruxolitinib and vorinostat leads to highly potent inhibitors of JAK2 and histone deacetylase 6 (HDAC6). Bioorg Med Chem Lett 28: 2636 -2640 (2018) Hanessian, S; Auzzas, L; Giannini, G; Marzi, M; Cabri, W; Barbarino, M; Vesci, L; Pisano, C Omega-alkoxy analogues of SAHA (vorinostat) as inhibitors of HDAC: a study of chain-length and stereochemical dependence. Bioorg Med Chem Lett 17: 6261 -5 (2007) Chu-Farseeva, YY; Mustafa, N; Poulsen, A; Tan, EC; Yen, JJY; Chng, WJ; Dymock, BW Design and synthesis of potent dual inhibitors of JAK2 and HDAC based on fusing the pharmacophores of XL019 and vorinostat. Eur J Med Chem 158: 593 -619 (2018) Cai, J; Wei, H; Hong, KH; Wu, X; Zong, X; Cao, M; Wang, P; Li, L; Sun, C; Chen, B; Zhou, G; Chen, J; Ji, M Discovery, bioactivity and docking simulation of Vorinostat analogues containing 1,2,4-oxadiazole moiety as potent histone deacetylase inhibitors and antitumor agents. Bioorg Med Chem 23: 3457 -71 (2015) Yao, L; Mustafa, N; Tan, EC; Poulsen, A; Singh, P; Duong-Thi, MD; Lee, JXT; Ramanujulu, PM; Chng, WJ; Yen, JJY; Ohlson, S; Dymock, BW Design and Synthesis of Ligand Efficient Dual Inhibitors of Janus Kinase (JAK) and Histone Deacetylase (HDAC) Based on Ruxolitinib and Vorinostat. J Med Chem 60: 8336 -8357 (2017)
ChEMBL_2069432 (CHEMBL4724685) Displacement of (1-(2-aminoethyl)-3,5-dimethyl-1H-pyrazol-4-yl)(4-((1-neopentyl-1H-benzo[d]imidazol-2-yl)methyl)piperazin-1-yl)methanone from AFG3L2 in vorinostat-stimulated human Jurkat 2C4 cells infected with latent HIV-1 by pull-down experiment based competitive binding assay ChEMBL_2069433 (CHEMBL4724686) Displacement of (1-(2-aminoethyl)-3,5-dimethyl-1H-pyrazol-4-yl)(4-((1-neopentyl-1H-benzo[d]imidazol-2-yl)methyl)piperazin-1-yl)methanone from NMRAL1 in vorinostat-stimulated human Jurkat 2C4 cells infected with latent HIV-1 by pull-down experiment based competitive binding assay ChEMBL_2069434 (CHEMBL4724687) Displacement of (1-(2-aminoethyl)-3,5-dimethyl-1H-pyrazol-4-yl)(4-((1-neopentyl-1H-benzo[d]imidazol-2-yl)methyl)piperazin-1-yl)methanone from FNTB in vorinostat-stimulated human Jurkat 2C4 cells infected with latent HIV-1 by pull-down experiment based competitive binding assay ChEMBL_2069435 (CHEMBL4724688) Displacement of (1-(2-aminoethyl)-3,5-dimethyl-1H-pyrazol-4-yl)(4-((1-neopentyl-1H-benzo[d]imidazol-2-yl)methyl)piperazin-1-yl)methanone from FNTA in vorinostat-stimulated human Jurkat 2C4 cells infected with latent HIV-1 by pull-down experiment based competitive binding assay Inhibitory Activity of the Compounds on HDAC6 Prepare a 10 mM stock solution of a compound with DMSO. Take 10 μl of stock solution and dilute with 90 μl DMSO to become a 1 mM working solution. The compound was three-fold serially diluted to total of 11 concentrations including a DMSO negative control. Add 3 μl of each concentration to 197 μl reaction buffer (20 mM Hepes pH 8.0, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 0.05% BSA, 0.5 mM TCEP), mix well and add 10 μl to a 384-well plate. In the final reaction system, the concentrations of the compound were 10 μM to 0.51 nM. Duplicate, and add 10 μl 3XHDAC solution (BPS, Cat. 50006, 0.3 nM) to each well, followed by incubating at 23° C. for 20 minutes. Then, add 3× substrate solution (Anaspec, Cat. 61855, 15 μM), centrifuge to mix, and incubate at 23° C. for 90 minutes. Add 30 μl trypsin/SAHA mixture (20 mM Hepes pH 8.0, 100 mM NaCl, 10 mM SAHA, 0.01 mg/ml trypsin), incubate at 23° C. for 60 minutes to terminate the reaction. Finally, read fluorescence data by Envision (390 nm excitation, 460 nm emission). A high value at 430 nm indicates high kinase activity, while low value at 430 nm indicates that the kinase activity was inhibited. Finally, analyze the data by XLfit5 software and calculate the IC50 value of the compound. Vorinostat (SAHA) was a positive reference compound.
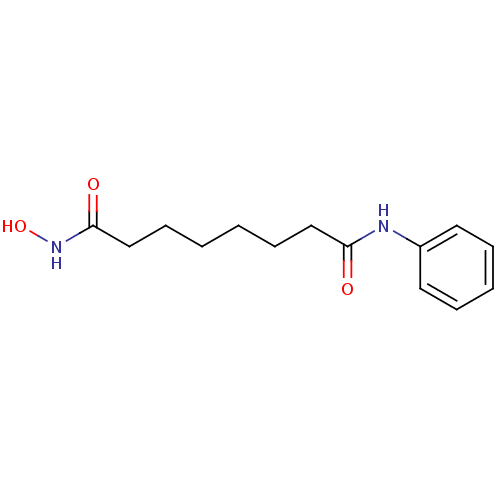 SAHA US11505523, Compound SAHA US9353061, SAHA BDBM19149 cid_5311 suberoylanilide hydroxamic acid N-hydroxy-N'-phenyloctanediamide US10011611, SAHA US9428447, SAHA US9695181, Vorinostat US10188756, Compound SAHA US11207431, SAHA Vorinostat US20240327418, Example SAHA US9115116, SAHA CHEMBL98 Zolinza
SAHA US11505523, Compound SAHA US9353061, SAHA BDBM19149 cid_5311 suberoylanilide hydroxamic acid N-hydroxy-N'-phenyloctanediamide US10011611, SAHA US9428447, SAHA US9695181, Vorinostat US10188756, Compound SAHA US11207431, SAHA Vorinostat US20240327418, Example SAHA US9115116, SAHA CHEMBL98 Zolinza