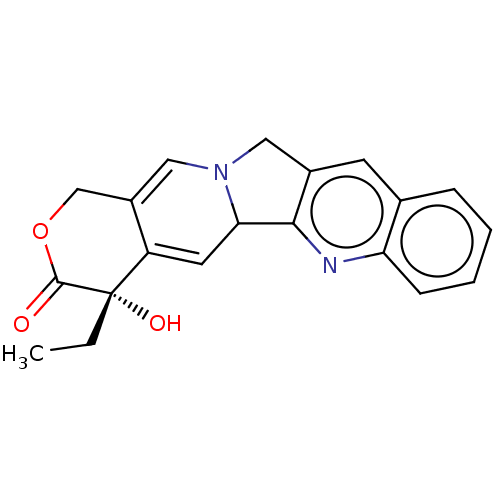
 BDBM246595 Camptothecin (CPT)
BDBM246595 Camptothecin (CPT)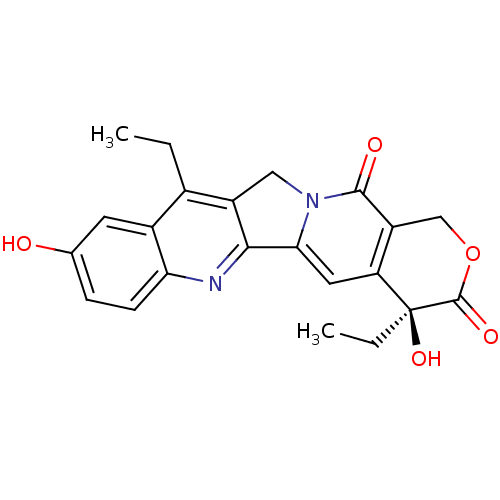 7-ETHYL-10-HYDROXY-CAMPTOTHECIN BDBM50418088
7-ETHYL-10-HYDROXY-CAMPTOTHECIN BDBM50418088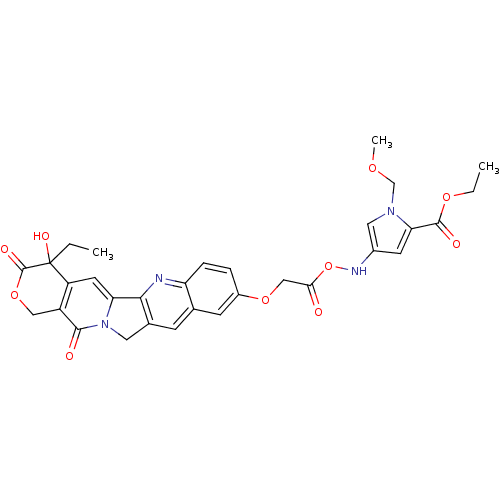 10-[[[1-(Methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL74459 BDBM50055650
10-[[[1-(Methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL74459 BDBM50055650 CHEMBL312690 10-[[[1-(Methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055653
CHEMBL312690 10-[[[1-(Methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055653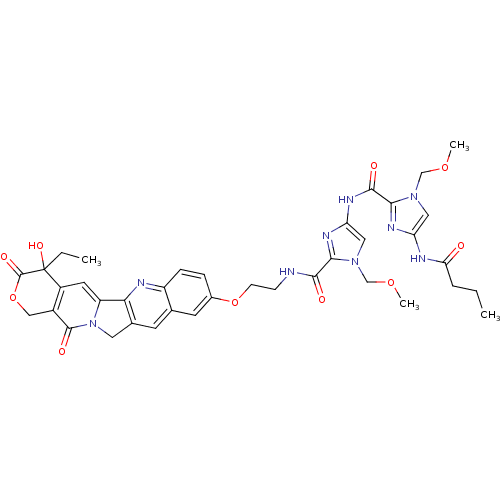 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidoimidazole-2-carboxamido]imidazole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL311855 BDBM50055656
10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidoimidazole-2-carboxamido]imidazole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL311855 BDBM50055656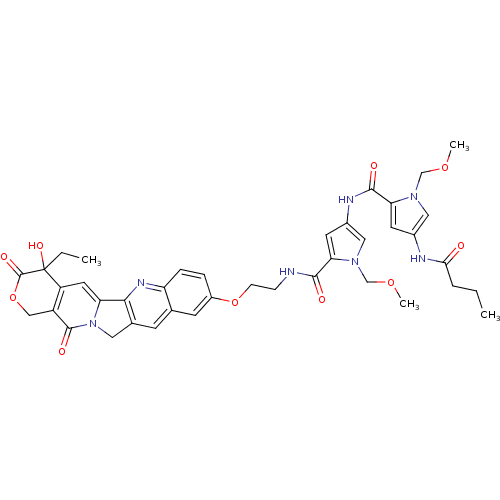 BDBM50055648 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL74406
BDBM50055648 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL74406 CHEMBL72353 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055652
CHEMBL72353 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055652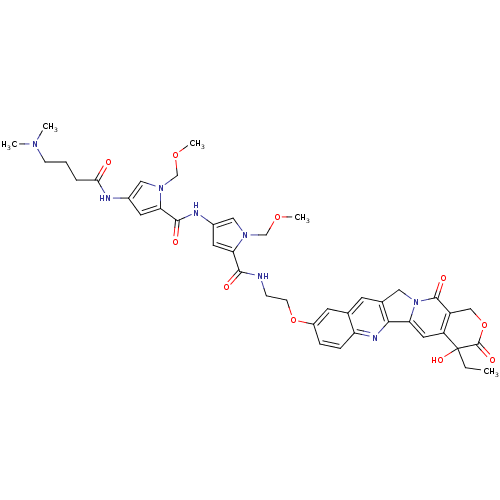 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin BDBM50055651 CHEMBL64874
10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin BDBM50055651 CHEMBL64874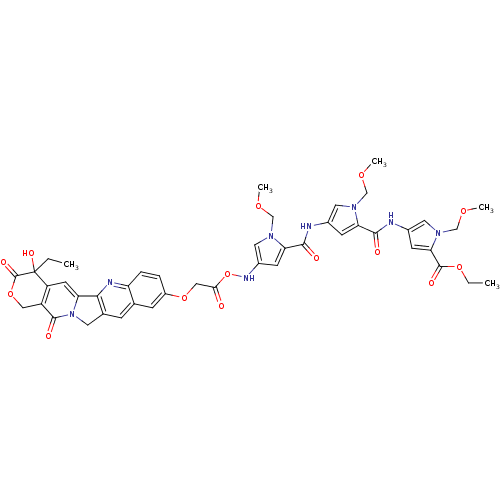 BDBM50055649 CHEMBL73002 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin
BDBM50055649 CHEMBL73002 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL266468 BDBM50055647 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin
CHEMBL266468 BDBM50055647 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin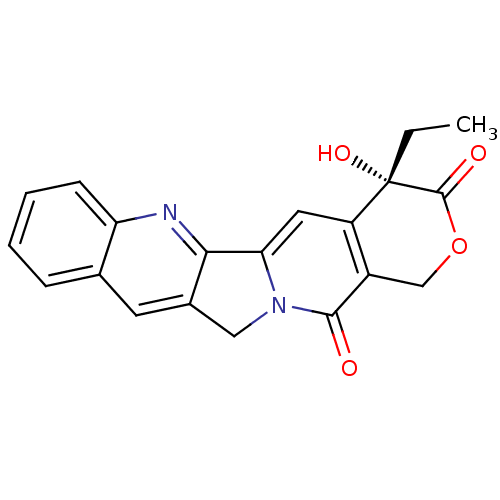 Camptothecin (CT) (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]florene-3,13-dione 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (CPT, Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4-ethyl-4-hydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione BDBM50008923 US20240246941, Compound SJ000285410 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin or CPT) camptothecine CHEMBL65 cid_24360
Camptothecin (CT) (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]florene-3,13-dione 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (CPT, Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4-ethyl-4-hydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione BDBM50008923 US20240246941, Compound SJ000285410 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin or CPT) camptothecine CHEMBL65 cid_24360 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL386919 BDBM50055663
10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL386919 BDBM50055663 4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione SN-38 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (SN-38) BDBM50092821 (S)-4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 7-ETHYL-10-HYDROXY-CAMPTOTHECIN (R)-4-Ethyl-4-methyl-1,4a,12,13a-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (S)-4,11-Diethyl-4,9-dihydroxy-1,5,5a,12-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione CHEMBL837 7-ethyl-10-hydroxycamptothecin 7-Ethyl-10-hydroxy-20(S)-camptothecin 4,11-diethyl-4,9-dihydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione
4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione SN-38 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (SN-38) BDBM50092821 (S)-4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 7-ETHYL-10-HYDROXY-CAMPTOTHECIN (R)-4-Ethyl-4-methyl-1,4a,12,13a-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (S)-4,11-Diethyl-4,9-dihydroxy-1,5,5a,12-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione CHEMBL837 7-ethyl-10-hydroxycamptothecin 7-Ethyl-10-hydroxy-20(S)-camptothecin 4,11-diethyl-4,9-dihydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione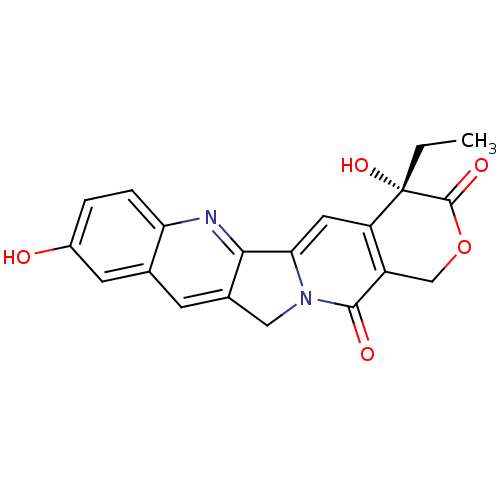 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (10-hydroxycamptothecin) 10-hydroxy-camptothecin CHEMBL273862 (20S)-4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-hydroxycamptothecin 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-Hydroxycamptothecine BDBM50008922 4-Ethyl-4,10-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (10-hydroxycamptothecin) 10-hydroxy-camptothecin CHEMBL273862 (20S)-4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-hydroxycamptothecin 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-Hydroxycamptothecine BDBM50008922 4-Ethyl-4,10-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
- Zhu, Q; Yu, X; Shen, Q; Zhang, Q; Su, M; Zhou, Y; Li, J; Chen, Y; Lu, W A series of camptothecin prodrugs exhibit HDAC inhibition activity. Bioorg Med Chem 26: 4706-4715 (2018)
- Lackey, K; Besterman, JM; Fletcher, W; Leitner, P; Morton, B; Sternbach, DD Rigid analogs of camptothecin as DNA topoisomerase I inhibitors. J Med Chem 38: 906-11 (1995)
- Niizuma, S; Tsukazaki, M; Suda, H; Murata, T; Ohwada, J; Ozawa, S; Fukuda, H; Murasaki, C; Kohchi, M; Morikami, K; Yoshinari, K; Endo, M; Ura, M; Tanimura, H; Miyazaki, Y; Takasuka, T; Kawashima, A; Nanba, E; Nakano, K; Ogawa, K; Kobayashi, K; Okabe, H; Umeda, I; Shimma, N Synthesis of new camptothecin analogs with improved antitumor activities. Bioorg Med Chem Lett 19: 2018-21 (2009)
- Dal Pozzo, A; Ni, MH; Esposito, E; Dallavalle, S; Musso, L; Bargiotti, A; Pisano, C; Vesci, L; Bucci, F; Castorina, M; Foderà, R; Giannini, G; Aulicino, C; Penco, S Novel tumor-targeted RGD peptide-camptothecin conjugates: synthesis and biological evaluation. Bioorg Med Chem 18: 64-72 (2010)
- Lackey, K; Sternbach, DD; Croom, DK; Emerson, DL; Evans, MG; Leitner, PL; Luzzio, MJ; McIntyre, G; Vuong, A; Yates, J; Besterman, JM Water soluble inhibitors of topoisomerase I: quaternary salt derivatives of camptothecin. J Med Chem 39: 713-9 (1996)
- Cincinelli, R; Musso, L; Artali, R; Guglielmi, M; Bianchino, E; Cardile, F; Colelli, F; Pisano, C; Dallavalle, S Camptothecin-psammaplin A hybrids as topoisomerase I and HDAC dual-action inhibitors. Eur J Med Chem 143: 2005-2014 (2018)
- Wall, ME; Wani, MC; Nicholas, AW; Manikumar, G; Tele, C; Moore, L; Truesdale, A; Leitner, P; Besterman, JM Plant antitumor agents. 30. Synthesis and structure activity of novel camptothecin analogs. J Med Chem 36: 2689-700 (1993)
- Kim, DK; Ryu, DH; Lee, JY; Lee, N; Kim, YW; Kim, JS; Chang, K; Im, GJ; Kim, TK; Choi, WS Synthesis and biological evaluation of novel A-ring modified hexacyclic camptothecin analogues. J Med Chem 44: 1594-602 (2001)
- Bedeschi, A; Zarini, F; Cabri, W; Candiani, I; Penco, S; Capolongo, L; Ciomei, M; Farao, M; Grandi, M Synthesis and antitumor activity of a new class of water soluble camptothecin derivatives Bioorg Med Chem Lett 6: 671-674 (1996)
- Peel, MR; Sternbach, DD The synthesis and evaluation of flexible analogues of the topoisomerase I inhibitor, camptothecin Bioorg Med Chem Lett 4: 2753-2758 (1994)
- Manikumar, G; Wadkins, RM; Bearss, D; Von Hoff, DD; Wani, MC; Wall, ME Camptothecin analogs with bulky, hydrophobic substituents at the 7-position via a Grignard reaction. Bioorg Med Chem Lett 14: 5377-81 (2004)
- Redinbo, MR Selective β-glucuronidase inhibitors as a treatment for side effects of camptothecin antineoplastic agents US Patent US9334288 (2016)
- Zakharenko, A; Luzina, O; Koval, O; Nilov, D; Gushchina, I; Dyrkheeva, N; Švedas, V; Salakhutdinov, N; Lavrik, O Tyrosyl-DNA Phosphodiesterase 1 Inhibitors: Usnic Acid Enamines Enhance the Cytotoxic Effect of Camptothecin. J Nat Prod 79: 2961-2967 (2016)
- Unleashing the Potential of Camptothecin: Exploring Innovative Strategies for Structural Modification and Therapeutic Advancements.
- Zhao, R; al-Said, NH; Sternbach, DL; Lown, JW Camptothecin and minor-groove binder hybrid molecules: synthesis, inhibition of topoisomerase I, and anticancer cytotoxicity in vitro. J Med Chem 40: 216-25 (1997)
- Luzzio, MJ; Besterman, JM; Emerson, DL; Evans, MG; Lackey, K; Leitner, PL; McIntyre, G; Morton, B; Myers, PL; Peel, M Synthesis and antitumor activity of novel water soluble derivatives of camptothecin as specific inhibitors of topoisomerase I. J Med Chem 38: 395-401 (1995)
- Manita, D; Toba, Y; Takakusagi, Y; Matsumoto, Y; Kusayanagi, T; Takakusagi, K; Tsukuda, S; Takada, K; Kanai, Y; Kamisuki, S; Sakaguchi, K; Sugawara, F Camptothecin (CPT) directly binds to human heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1) and inhibits the hnRNP A1/topoisomerase I interaction. Bioorg Med Chem 19: 7690-7 (2011)
- Chu, XY; Kato, Y; Niinuma, K; Sudo, KI; Hakusui, H; Sugiyama, Y Multispecific organic anion transporter is responsible for the biliary excretion of the camptothecin derivative irinotecan and its metabolites in rats. J Pharmacol Exp Ther 281: 304-14 (1997)
- Zhou, J; Bian, X; Kan, Z; Cai, Z; Jiang, Y; Wang, Z; Li, Y; Shi, W; Qian, H Exploration and Biological Evaluation of Bispecific Peptides Derived from Anti-HER2 Antibodies and Peptide-Camptothecin Conjugates for HER2-Positive Breast Cancer. J Med Chem 65: 15123-15139 (2022)
- ChEMBL_424834 (CHEMBL908461) Release of camptothecin-induced cell cycle arrest in NCI-H1299 cells mediated by CHEK1 inhibition
- ChEMBL_834717 (CHEMBL2072693) Inhibition of CHK1 in human HT29 cells assessed as abrogation of camptothecin induced check point
- ChEMBL_1686964 (CHEMBL4037443) Inhibition of CHK1 in human HT29 cells assessed as abrogation of camptothecin-induced G2/M phase arrest
- Inhibition Effect of the Compounds of the Present Invention on the Enzyme Activity of CYP2D6 in Human Liver Microsome Table 3: I. Experimental Materials and Instruments1. Phosphate buffer solution (PBS);2. NADPH (Sigma N-1630);3. Human liver microsome (Corning Gentest);4. ABI QTrap 4000 liquid chromatograph/mass spectrometer (AB Sciex);5. Inertsil C8-3 column, 4.6×50 mm, 5 μm (Dikma Technologies Inc., USA); and6. CYP probe substrate (20 μM dextromethorphan, SIGMA Q0750) and positive control inhibitor (quinidine, SIGMA D9684).II. Experimental Procedures100 mM PBS buffer was formulated, which was then used to formulate 2.5 mg/ml human microsome solution and 5 mM NADPH solution. The 5× concentration of the compound working solution was diluted with PBS in gradients (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM). The 5× concentration of quinidine working solution was diluted with PBS in gradients (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM). Dextromethorphan working solution was diluted with PBS to a concentration of 20 μM.20 μl of 2.5 mg/ml microsome solution, 20 μl of 20 μM dextromethorphan working solution, 20 μl of MgCl2 solution and 20 μl of the compound working solution (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM, different reaction systems for each concentration) were mixed well. For the positive control group, the compound was replaced with the same concentration of quinidine. The mixture together with 5 mM NADPH solution was pre-incubated at 37° C. for 5 minutes. After 5 minutes, 20 μl of NADPH was added to each well, the reaction was initiated, and the plate was incubated for 30 minutes. All the incubated samples were present in duplicate. After 30 minutes, 250 μl of acetonitrile containing internal standard (100 ng/ml camptothecin) was added to all samples, mixed well, shaken at 800 rpm for 10 minutes, and then centrifuged at 3700 rpm for 10 minutes. 80 μl of the supernatant was taken and analyzed by LC-MS/MS.
- Inhibition Effect of the Compounds of the Present Invention on the Enzyme Activity of Midazolam Metabolite Site of CYP3A4 Table 2: I. Experimental Materials and Instruments1. Phosphate buffer solution (PBS) (Shanghai Basalmedia Technologies Co., Ltd., B320, similarly hereinafter);2. NADPH (Sigma N-1630);3. Human liver microsome (Corning Gentest);4. ABI QTrap 4000 liquid chromatograph/mass spectrometer (AB Sciex);5. Inertsil C8-3 column, 4.6×50 mm, 5 μm (Dikma Technologies Inc., USA); and6. CYP probe substrate (15 μM midazolam, SIGMA UC429) and positive control inhibitor (ketoconazole, SIGMA K1003).II. Experimental Procedures100 mM PBS buffer was formulated, which was then used to formulate 2.5 mg/ml human microsome solution and 5 mM NADPH solution. The 5× concentration of the compound working solution was diluted with PBS in gradients (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM). The 5× concentration of ketoconazole working solution was diluted with PBS in gradients (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM). Midazolam working solution was diluted with PBS to a concentration of 15 μM.20 μl of 2.5 mg/ml microsome solution, 20 μl of 15 μM midazolam working solution, 20 μl of MgCl2 solution and 20 μl of the compound working solution (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM, different reaction systems for each concentration) were mixed well. For the positive control group, the compound was replaced with the same concentration of ketoconazole. The mixture together with 5 mM NADPH solution was pre-incubated at 37° C. for 5 minutes. After 5 minutes, 20 μl of NADPH was added to each well, the reaction was initiated, and the plate was incubated for 30 minutes. All the incubated samples were present in duplicate. After 30 minutes, 250 μl of acetonitrile containing internal standard (100 ng/ml camptothecin) was added to all samples, mixed well, shaken at 800 rpm for 10 minutes, and then centrifuged at 3700 rpm for 10 minutes. 80 μl of the supernatant was taken and analyzed by LC-MS/MS.
- Inhibition Effect of the Compounds of the Present Invention on the Enzyme Activity of Testosterone Metabolite Site of CYP3A4 in Human Liver Microsomes Table 4: I. Experimental Materials and Instruments1. Phosphate buffer solution (PBS);2. NADPH (Sigma N-1630);3. Human liver microsome (Corning Gentest);4. ABI QTrap 4000 liquid chromatograph/mass spectrometer (AB Sciex);5. Inertsil C8-3 column, 4.6×50 mm, 5 μm (Dikma Technologies Inc., USA); and6. CYP probe substrate (testosterone/100 μM, SIGMA K1003) and positive control inhibitor (ketoconazole, Dr. Ehrenstorfer GmbH, C17322500).II. Experimental Procedures100 mM PBS buffer was formulated, which was then used to formulate 2.5 mg/ml human microsome solution and 5 mM NADPH solution. The 5× concentration of the compound working solution was diluted with PBS in gradients (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM). The 5× concentration of ketoconazole working solution was diluted with PBS in gradients (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM). Dextromethorphan working solution was diluted with PBS to a concentration of 50 μM.20 μl of 2.5 mg/ml microsome solution, 20 μl of 50 μM testosterone working solution, 20 μl of MgCl2 solution and 20 μl of the compound working solution (150, 50, 15, 5, 1.5, 0.15, 0.015, 0 μM, different reaction systems for each concentration) were mixed well. For the positive control group, the compound was replaced with the same concentration of ketoconazole. The mixture together with 5 mM NADPH solution was pre-incubated at 37° C. for 5 minutes. After 5 minutes, 20 μl of NADPH was added to each well, the reaction was initiated, and the plate was incubated for 30 minutes. All the incubated samples were present in duplicate. After 30 minutes, 250 μl of acetonitrile containing internal standard (100 ng/ml camptothecin) was added to all samples, mixed well, shaken at 800 rpm for 10 minutes, and then centrifuged at 3700 rpm for 10 minutes. 80 μl of the supernatant was taken and analyzed by LC-MS/MS.
 BDBM246595 Camptothecin (CPT)
BDBM246595 Camptothecin (CPT) 7-ETHYL-10-HYDROXY-CAMPTOTHECIN BDBM50418088
7-ETHYL-10-HYDROXY-CAMPTOTHECIN BDBM50418088 10-[[[1-(Methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL74459 BDBM50055650
10-[[[1-(Methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL74459 BDBM50055650 CHEMBL312690 10-[[[1-(Methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055653
CHEMBL312690 10-[[[1-(Methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055653 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidoimidazole-2-carboxamido]imidazole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL311855 BDBM50055656
10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidoimidazole-2-carboxamido]imidazole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL311855 BDBM50055656 BDBM50055648 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL74406
BDBM50055648 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin CHEMBL74406 CHEMBL72353 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055652
CHEMBL72353 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin BDBM50055652 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin BDBM50055651 CHEMBL64874
10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin BDBM50055651 CHEMBL64874 BDBM50055649 CHEMBL73002 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin
BDBM50055649 CHEMBL73002 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-carbethoxypyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL266468 BDBM50055647 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin
CHEMBL266468 BDBM50055647 10-[2-[1-(Methoxymethyl)-4-[1-(methoxymethyl)-4-[1-(methoxymethyl)-4-[4-(dimethylamino)butyramidopyrrole-2-carboxamido]pyrrole-2-carboxamido]pyrrole-2-carboxamido]ethoxy]-20(R,S)-camptothecin Camptothecin (CT) (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]florene-3,13-dione 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (CPT, Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4-ethyl-4-hydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione BDBM50008923 US20240246941, Compound SJ000285410 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin or CPT) camptothecine CHEMBL65 cid_24360
Camptothecin (CT) (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]florene-3,13-dione 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (CPT, Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4-ethyl-4-hydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione (S)-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione BDBM50008923 US20240246941, Compound SJ000285410 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (Camptothecin) 4-Ethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (camptothecin or CPT) camptothecine CHEMBL65 cid_24360 10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL386919 BDBM50055663
10-[[[1-(Methoxymethyl)-2-[[1-(methoxymethyl)-2-[[1-(methoxymethyl)-2-[[3-(dimethylamino)propyl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]pyrrol-4-yl]carbamoyl]methoxy]-2(R,S)-camptothecin CHEMBL386919 BDBM50055663 4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione SN-38 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (SN-38) BDBM50092821 (S)-4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 7-ETHYL-10-HYDROXY-CAMPTOTHECIN (R)-4-Ethyl-4-methyl-1,4a,12,13a-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (S)-4,11-Diethyl-4,9-dihydroxy-1,5,5a,12-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione CHEMBL837 7-ethyl-10-hydroxycamptothecin 7-Ethyl-10-hydroxy-20(S)-camptothecin 4,11-diethyl-4,9-dihydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione
4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione SN-38 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (SN-38) BDBM50092821 (S)-4,11-Diethyl-4-hydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 4,11-Diethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 7-ETHYL-10-HYDROXY-CAMPTOTHECIN (R)-4-Ethyl-4-methyl-1,4a,12,13a-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (S)-4,11-Diethyl-4,9-dihydroxy-1,5,5a,12-tetrahydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione CHEMBL837 7-ethyl-10-hydroxycamptothecin 7-Ethyl-10-hydroxy-20(S)-camptothecin 4,11-diethyl-4,9-dihydroxy-(4S)-3,4,12,14-tetrahydro-1H-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14-dione 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (10-hydroxycamptothecin) 10-hydroxy-camptothecin CHEMBL273862 (20S)-4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-hydroxycamptothecin 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-Hydroxycamptothecine BDBM50008922 4-Ethyl-4,10-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione
4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione (10-hydroxycamptothecin) 10-hydroxy-camptothecin CHEMBL273862 (20S)-4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-hydroxycamptothecin 4-Ethyl-4,9-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione 10-Hydroxycamptothecine BDBM50008922 4-Ethyl-4,10-dihydroxy-1,12-dihydro-4H-2-oxa-6,12a-diaza-dibenzo[b,h]fluorene-3,13-dione