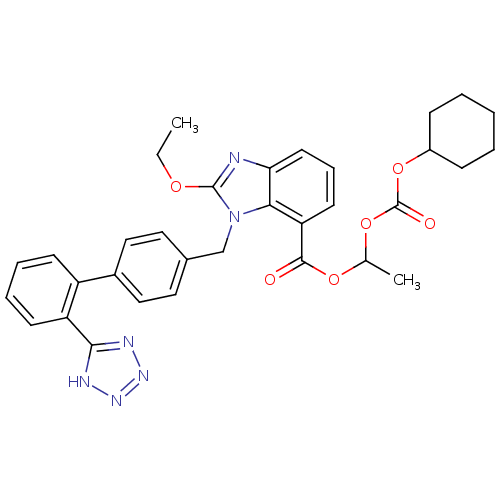
 BDBM50318907 TCV-116 Candesartan Blopress candesartancilexetil Amias Atacand Kenzen CHEMBL1014 CANDESARTAN CILEXETIL 2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester Parapres
BDBM50318907 TCV-116 Candesartan Blopress candesartancilexetil Amias Atacand Kenzen CHEMBL1014 CANDESARTAN CILEXETIL 2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester Parapres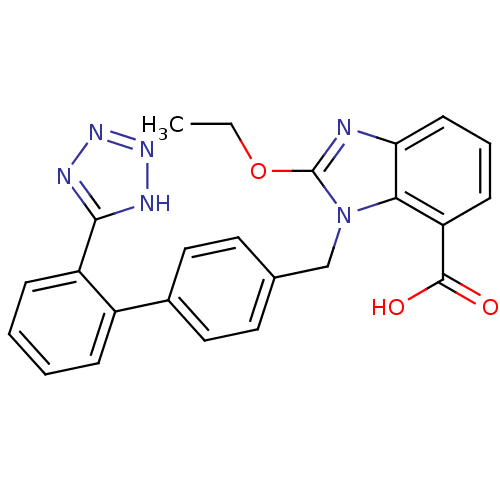 2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid(CV-11974) 2-ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid CHEMBL1016 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid BDBM50240609 CV-11974 CANDESARTAN
2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid(CV-11974) 2-ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid CHEMBL1016 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid BDBM50240609 CV-11974 CANDESARTAN
- Ni, S; Chen, X; Yu, Q; Xu, Y; Hu, Z; Zhang, J; Zhang, W; Li, B; Yang, X; Mao, F; Huang, J; Sun, Y; Li, J; Jia, L Discovery of candesartan cilexetic as a novel neddylation inhibitor for suppressing tumor growth. Eur J Med Chem 185: (2020)
- Radioligand Assay All synthesized ligands were evaluated in a radioligand assay by displacing 125I-[Sar1, Ile8]-Angiotensin II (Perkin Elmer, NEX248050UC) from human AT2R fused to Cb23 or human/rodent/cynomolgus/minipig/dog WT AT2R in HEK-293 cells membrane preparations, using C21 (Vicore Pharma) and Angiotensin II (endogenous ligand) as reference. The affinity was determined using an eight-point dose-response curve, each point performed in duplicates. The compounds were also evaluated in a counterscreen binding assay for displacement of 125I-[Sar1, Ile8]-Angiotensin II binding to human AT1R in HEK-293 cell membranes. For AT1R, the percent displacement was determined at 1 μM and 10 M (in duplicates) or using an eight-point dose-response curve, each point performed in duplicates with Candesartan and Losartan used as reference.For the AT2R/AT1R binding assays, cell membranes, expressing AT2R_Cb23, AT2R or AT1R, were incubated with 0.05 nM 125I-[Sarl, Ile8]-Ang II. The ligand competition assay was performed in a total volume of 100 μL assay buffer (50 mM Tris, 5 mM MgCl2, 1 mM EDTA, 0.1% BSA, pH 7.4), at concentrations ranging from 1 μM to 10 μM. For each experiment, each ligand concentration was tested in duplicate. Non-specific binding (NSB) was determined by the inclusion of 10 μM unlabeled [Sarl]-Ang II (Sigma Aldrich). The reaction was initiated by the addition of radioligand, after which the plates were incubated at 25° C. for one hour. The reaction was terminated by rapid filtration using a vacuum harvester, applying six washes with 100 μL of ice-cold wash buffer (50 mM Tris.HCl, pH 7.4). The filter plates GF/C (Perkin Elmer) were pre-soaked in 0.5% PEI. The residual amount of radioactivity was determined via liquid scintillation counting. IC50 values, representing the concentration at which each ligand displaced 50% of 125I-[Sarl, Ile8]-Ang II, were calculated using GraphPad Prism 7.02 by applying a Non-linear regression equation (variable slope, four parameters) on the data.
 BDBM50318907 TCV-116 Candesartan Blopress candesartancilexetil Amias Atacand Kenzen CHEMBL1014 CANDESARTAN CILEXETIL 2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester Parapres
BDBM50318907 TCV-116 Candesartan Blopress candesartancilexetil Amias Atacand Kenzen CHEMBL1014 CANDESARTAN CILEXETIL 2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid 1-cyclohexyloxycarbonyloxy-ethyl ester Parapres 2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid(CV-11974) 2-ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid CHEMBL1016 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid BDBM50240609 CV-11974 CANDESARTAN
2-Ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid(CV-11974) 2-ethoxy-3-[2'-(1H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid CHEMBL1016 2-Ethoxy-3-[2'-(2H-tetrazol-5-yl)-biphenyl-4-ylmethyl]-3H-benzoimidazole-4-carboxylic acid BDBM50240609 CV-11974 CANDESARTAN