 GW-282974X BDBM50311471 Lapatinib ditosylate Lapatinib CHEMBL1076241
GW-282974X BDBM50311471 Lapatinib ditosylate Lapatinib CHEMBL1076241 LAPATINIB BDBM32368
LAPATINIB BDBM32368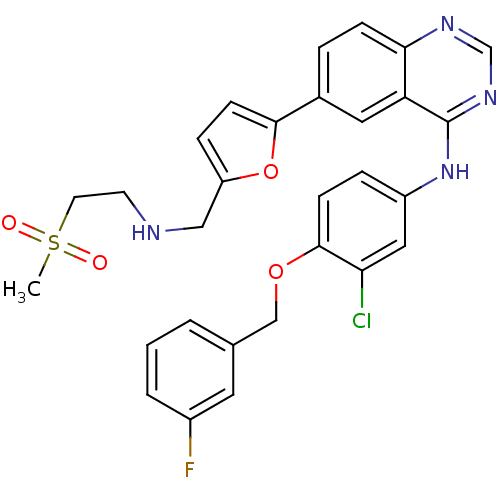 N-{3-chloro-4-[(3-fluoro-benzyl)oxy]phenyl}-6-[5-({2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine US9730934, Lapatinib ditosylate N-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-(5-{[(2-methanesulfonylethyl)amino]methyl}furan-2-yl)quinazolin-4-amine GW572016 BDBM5445 cid_208908 LAPATINIB DITOSYLATE US10507209, Compound Lapatinib CHEMBL554 Lapatinib Tykerb US10822334, Compound Lapatinib
N-{3-chloro-4-[(3-fluoro-benzyl)oxy]phenyl}-6-[5-({2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine US9730934, Lapatinib ditosylate N-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-(5-{[(2-methanesulfonylethyl)amino]methyl}furan-2-yl)quinazolin-4-amine GW572016 BDBM5445 cid_208908 LAPATINIB DITOSYLATE US10507209, Compound Lapatinib CHEMBL554 Lapatinib Tykerb US10822334, Compound Lapatinib
- Chan, EC; New, LS; Chua, TB; Yap, CW; Ho, HK; Nelson, SD Interaction of lapatinib with cytochrome P450 3A5. Drug Metab Dispos 40: 1414-22 (2012)
- Qiu, J; Liu, Q; Li, P; Jiang, Q; Chen, W; Li, D; Li, G; Shan, G Ligand-Directed Photodegradation of Interacting Proteins: Oxidative HER2/HER3 Heterodimer Degradation with a Lapatinib-Derived Photosensitizer. J Med Chem 66: 10265-10272 (2023)
- Lyu, A; Fang, L; Gou, S Design and synthesis of Lapatinib derivatives containing a branched side chain as HER1/HER2 targeting antitumor drug candidates. Eur J Med Chem 87: 631-42 (2014)
- Wang, Y; Lv, Z; Chen, F; Wang, X; Gou, S Conjugates Derived from Lapatinib Derivatives with Cancer Cell Stemness Inhibitors Effectively Reversed Drug Resistance in Triple-Negative Breast Cancer. J Med Chem 64: 12877-12892 (2021)
- Mahboobi, S; Sellmer, A; Winkler, M; Eichhorn, E; Pongratz, H; Ciossek, T; Baer, T; Maier, T; Beckers, T Novel chimeric histone deacetylase inhibitors: a series of lapatinib hybrides as potent inhibitors of epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER2), and histone deacetylase activity. J Med Chem 53: 8546-55 (2010)
- ChEMBL_1580716 (CHEMBL3811321) Inhibition of AXL in lapatinib-sensitive human BT474 cells
- ChEMBL_2147302 (CHEMBL5031648) Inhibition of ALDH1A1 in human drug-tolerant MDA-MB-231/lapatinib cells assessed as after 24 hrs
- In Vitro Enzyme Inhibitory Activity Samples:Controls: Gefitinib, erlotinib hydrochloride, purchased from Anqing worldchem Co., LTD.; lapatinib ditosylate, purchased from Taizhou Xingcheng Chempharm Co., Ltd.; CI-1033 hydrochloride, purchased from Shanghai hanxiangchem, Co., Ltd.; andThe present compounds: lab-made, their chemical names and structural formulae are shown in the preparation examples.Assay Procedures:The abbreviations used in the following assay have the following meanings:HEPES: hydroxyethyl piperazine ethanesulfonic acid;Brij-35: polyoxyethylene lauryl ether;DTT: dithiothreitol;Coating Reagent #3: #3 coating agent;EDTA: ethylene diamine tetraacetic acid, purchased from Sigma Co. Ltd.;FAM labeled peptide: fluorescein labeled peptide 22 (GL Biochem);ATP: adenosine triphosphate (Sigma);DMSO: dimethyl sulfoxide;EGFR: human epidermal growth factor receptor (Carna);HER2: human epidermal growth factor receptor 2 (Carna);HER4: human epidermal growth factor receptor 4 (Carna).1. Formulating the agents to be used in the assay(1) 1.25-fold MnCl2-free kinase buffer (62.5 mM HEPES, PH 7.5, 0.001875% Brij-35, 12.5 mM MgCl2, 2.5 mM DTT);(2) 1.25-fold MnCl2-containing kinase buffer (62.5 mM HEPES, pH 7.5, 0.001875% Brij-35, 12.5 mM MgCl2, 12.5 mM MnCl2, 2.5 mM DTT);(3) Stop buffer (100 mM HEPES, pH 7.5, 0.015% Brij-35, 0.2% Coating Reagent #3, 50 mM EDTA);(4) 2.5-fold kinase solutions (to the 1.25-fold kinase buffers were added the corresponding kinases to formulate 2.5-fold EGFR, HER2, HER4 kinase solutions);(5) 2.5-fold peptide solutions (to the 1.25-fold kinase buffers were added FAM labeled peptide and ATP to formulate the peptide solutions);(6) 5-fold compound solutions (using 100% DMSO to formulate 50-fold compound solutions having different concentration gradients, and diluting with water by 10 times to obtain 5-fold compound solutions having different concentration gradients);2. Adding 5 μL of a 5-fold compound solution to a 384-well plate;3. Adding 10 μL of a 2.5-fold kinase solution to incubate for 10 min;4. Then adding 10 μL of a 2.5-fold peptide solution, and reacting at 28° C. for 1 h; and5. Finally, adding 25 μL of stop buffer to terminate the reaction, and reading the data with Caliper.6. Curve fitting to obtain an IC50 value.The calculated inhibition ratio (%)=(the maximum conversion rate−the conversion rate)/(the maximum conversion rate−the minimum conversion rate)×100The curve fitting was conducted with the Xlfit software to obtain IC50 values.
 GW-282974X BDBM50311471 Lapatinib ditosylate Lapatinib CHEMBL1076241
GW-282974X BDBM50311471 Lapatinib ditosylate Lapatinib CHEMBL1076241 LAPATINIB BDBM32368
LAPATINIB BDBM32368 N-{3-chloro-4-[(3-fluoro-benzyl)oxy]phenyl}-6-[5-({2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine US9730934, Lapatinib ditosylate N-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-(5-{[(2-methanesulfonylethyl)amino]methyl}furan-2-yl)quinazolin-4-amine GW572016 BDBM5445 cid_208908 LAPATINIB DITOSYLATE US10507209, Compound Lapatinib CHEMBL554 Lapatinib Tykerb US10822334, Compound Lapatinib
N-{3-chloro-4-[(3-fluoro-benzyl)oxy]phenyl}-6-[5-({2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine US9730934, Lapatinib ditosylate N-{3-chloro-4-[(3-fluorophenyl)methoxy]phenyl}-6-(5-{[(2-methanesulfonylethyl)amino]methyl}furan-2-yl)quinazolin-4-amine GW572016 BDBM5445 cid_208908 LAPATINIB DITOSYLATE US10507209, Compound Lapatinib CHEMBL554 Lapatinib Tykerb US10822334, Compound Lapatinib