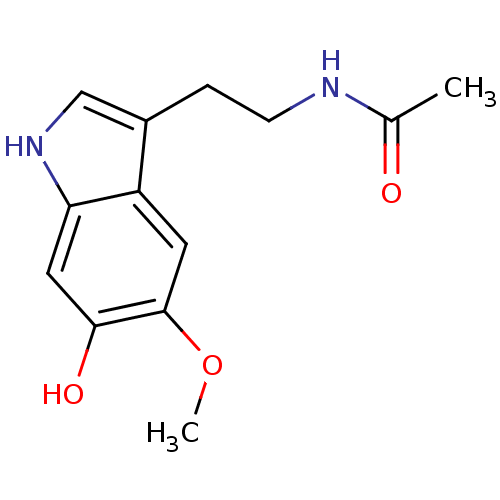
 BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy
BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy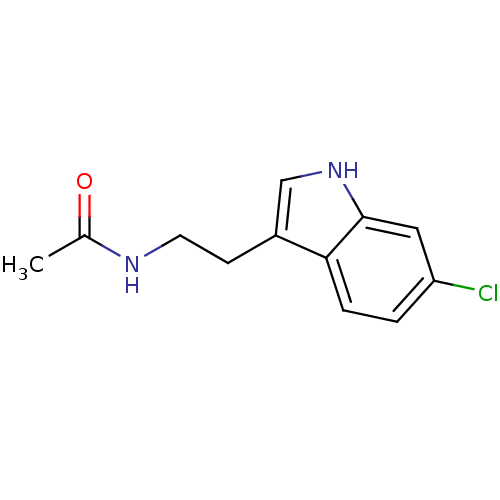 BDBM85063 Melatonin,6-Cl
BDBM85063 Melatonin,6-Cl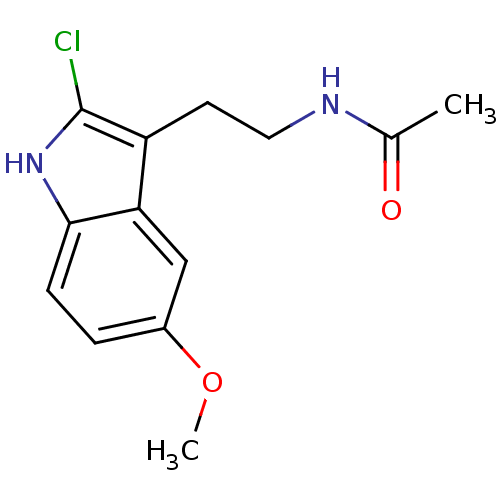 Melatonin,2-Chloro BDBM85236
Melatonin,2-Chloro BDBM85236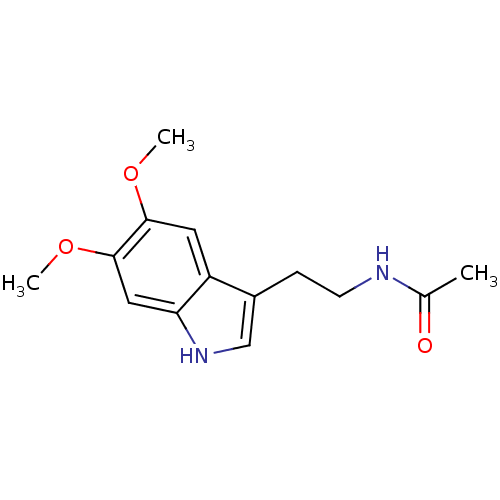 melatonin, 6-Methoxy BDBM82556
melatonin, 6-Methoxy BDBM82556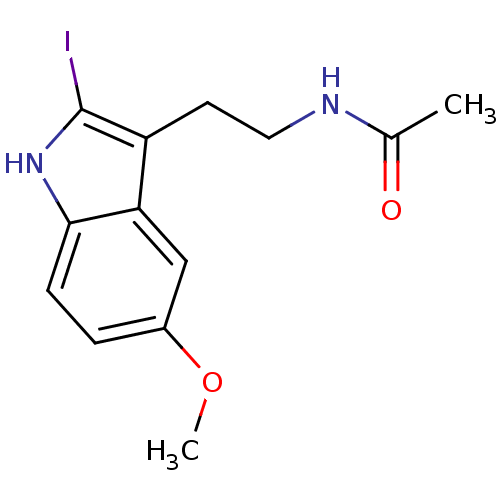 BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo
BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo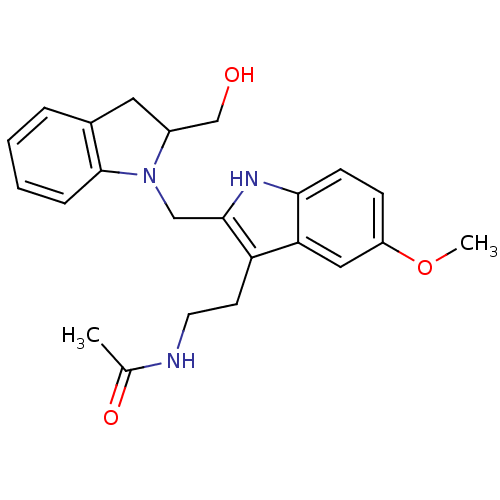 CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin
CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me
BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622
CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622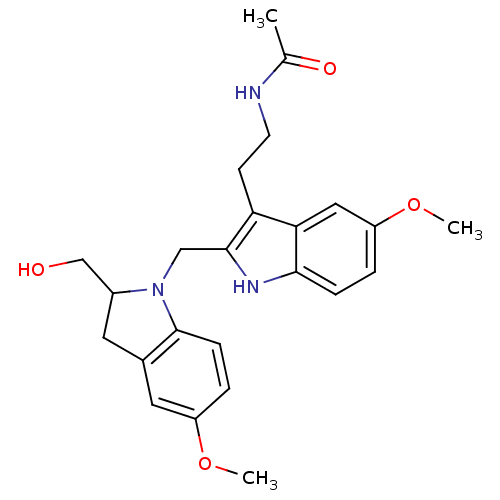 BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin
BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4
BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4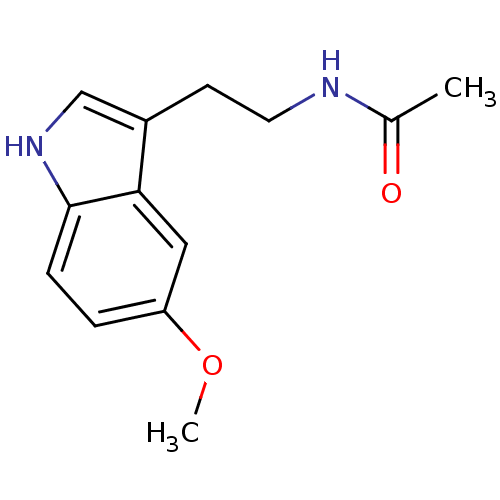 BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide
BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide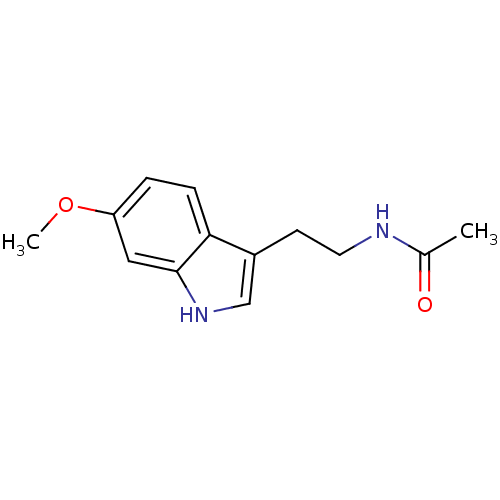 BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy
BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy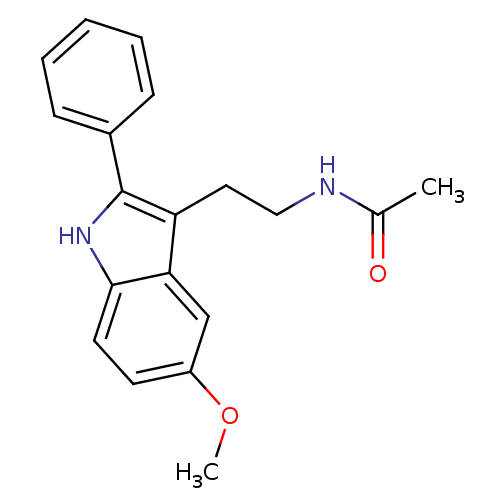 Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110
Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110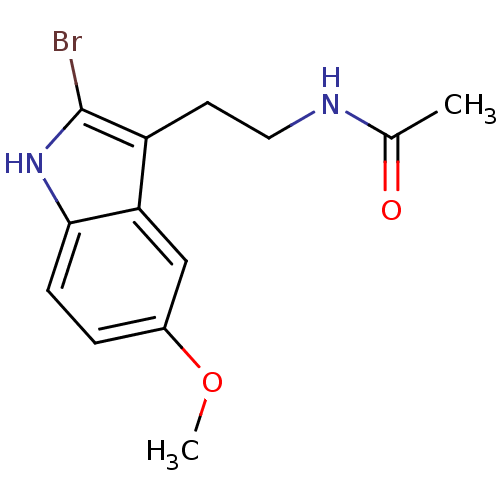 CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin)
CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin) N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730
N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a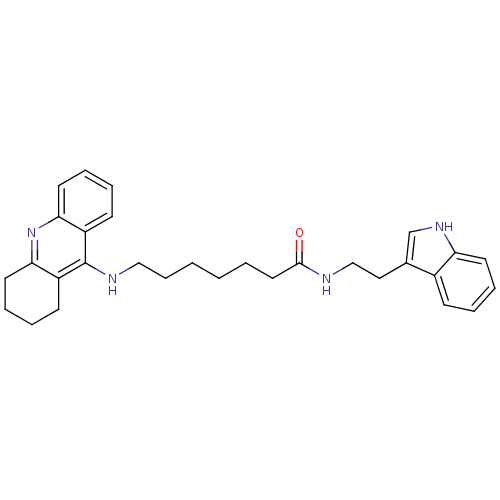 BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide
BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide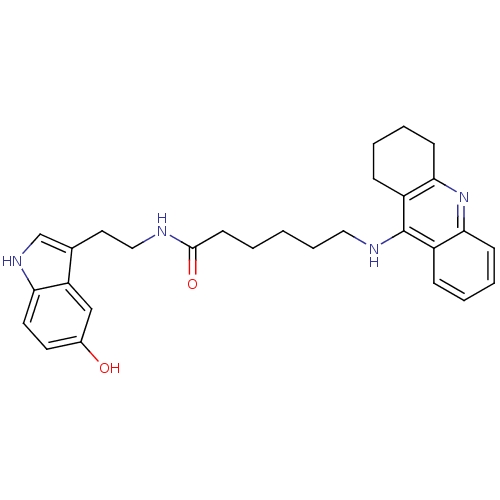 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide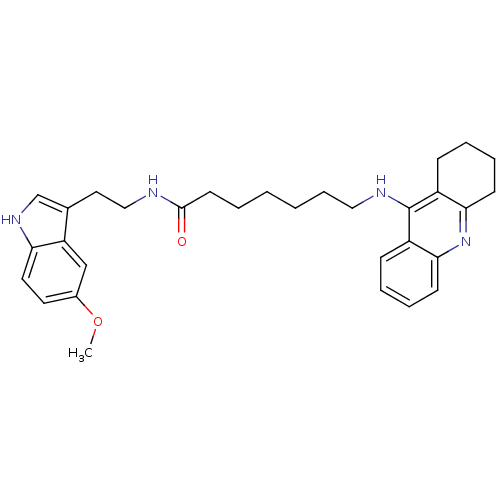 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014
N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014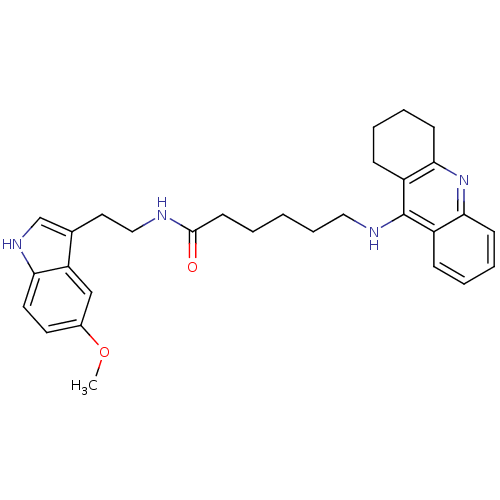 Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide
Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide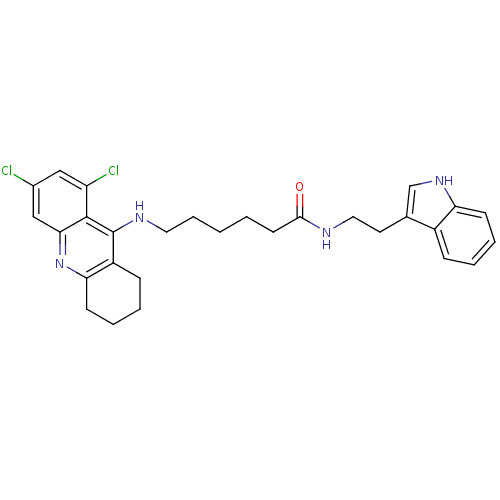 6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a
6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a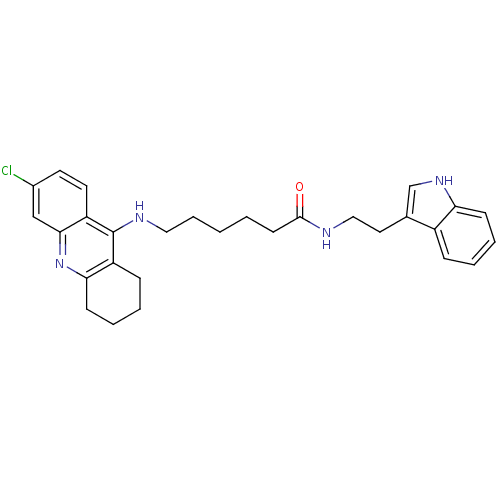 BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a
BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a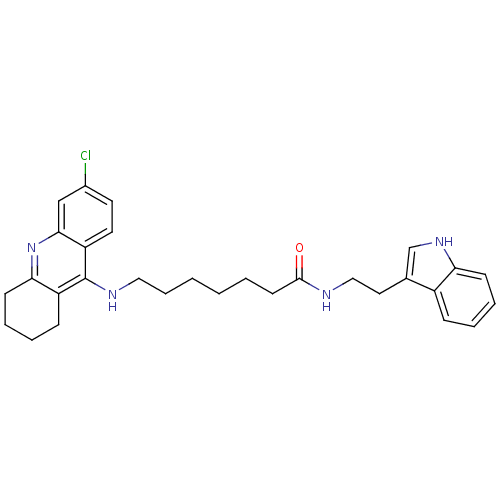 BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide
BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide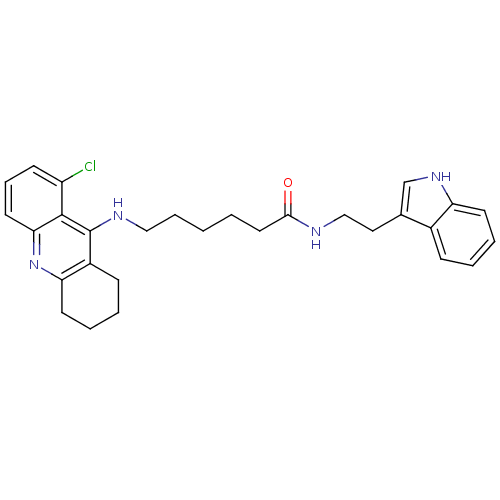 Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010
Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010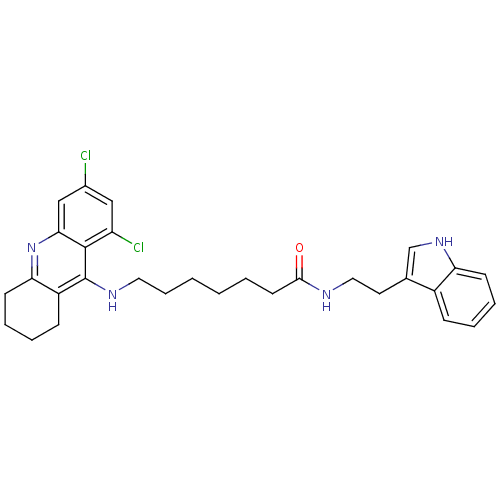 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide
7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide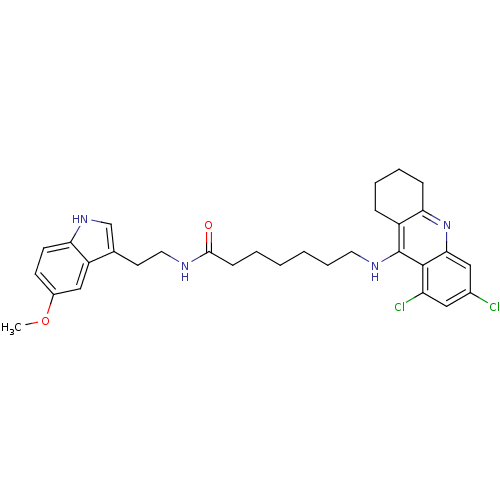 BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide
BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide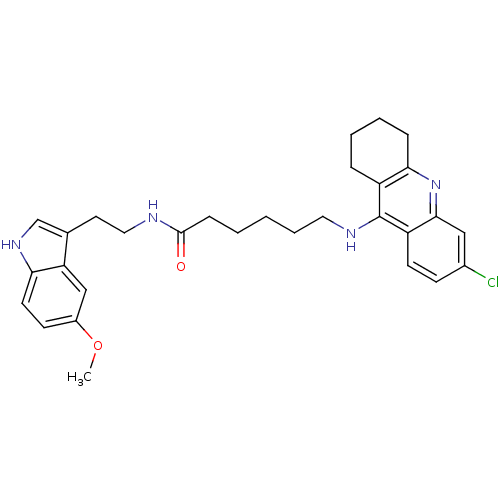 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a
6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a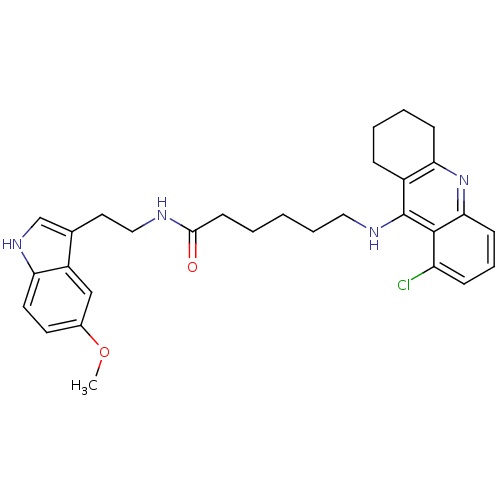 BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide
BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide
- Sugden, D; Pickering, H; Teh, MT; Garratt, PJ Melatonin receptor pharmacology: toward subtype specificity. Biol Cell 89: 531-7 (1997)
- Mor, M; Rivara, S; Silva, C; Bordi, F; Plazzi, PV; Spadoni, G; Diamantini, G; Balsamini, C; Tarzia, G; Fraschini, F; Lucini, V; Nonno, R; Stankov, BM Melatonin receptor ligands: synthesis of new melatonin derivatives and comprehensive comparative molecular field analysis (CoMFA) study. J Med Chem 41: 3831-44 (1998)
- Garratt, PJ; Jones, R; Tocher, DA; Sugden, D Mapping the melatonin receptor. 3. Design and synthesis of melatonin agonists and antagonists derived from 2-phenyltryptamines. J Med Chem 38: 1132-9 (1995)
- Reppert, SM; Godson, C; Mahle, CD; Weaver, DR; Slaugenhaupt, SA; Gusella, JF Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A 92: 8734-8 (1995)
- Karageorge, GN; Bertenshaw, S; Iben, L; Xu, C; Sarbin, N; Gentile, A; Dubowchik, GM Tetrahydroisoquinoline derivatives as melatonin MT2 receptor antagonists. Bioorg Med Chem Lett 14: 5881-4 (2004)
- Zlotos, DP; Attia, MI; Julius, J; Sethi, S; Witt-Enderby, PA 2-[(2,3-dihydro-1H-indol-1-yl)methyl]melatonin analogues: a novel class of MT2-selective melatonin receptor antagonists. J Med Chem 52: 826-33 (2009)
- Davies, DJ; Garratt, PJ; Tocher, DA; Vonhoff, S; Davies, J; Teh, MT; Sugden, D Mapping the melatonin receptor. 5. Melatonin agonists and antagonists derived from tetrahydrocyclopent[b]indoles, tetrahydrocarbazoles and hexahydrocyclohept[b]indoles. J Med Chem 41: 451-67 (1998)
- Morgan, PJ; Barrett, P; Howell, HE; Helliwell, R Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int 24: 101-46 (1994)
- Li, PK; Chu, GH; Gillen, ML; Parekh, T; Witt-Enderby, PA The development of a charged melatonin receptor ligand Bioorg Med Chem Lett 7: 2409-2414 (1997)
- Spadoni, G; Balsamini, C; Diamantini, G; Di Giacomo, B; Tarzia, G; Mor, M; Plazzi, PV; Rivara, S; Lucini, V; Nonno, R; Pannacci, M; Fraschini, F; Stankov, BM Conformationally restrained melatonin analogues: synthesis, binding affinity for the melatonin receptor, evaluation of the biological activity, and molecular modeling study. J Med Chem 40: 1990-2002 (1997)
- Epperson, JR; Deskus, JA; Gentile, AJ; Iben, LG; Ryan, E; Sarbin, NS 4-Substituted anilides as selective melatonin MT2 receptor agonists. Bioorg Med Chem Lett 14: 1023-6 (2004)
- Beresford, IJ; Browning, C; Starkey, SJ; Brown, J; Foord, SM; Coughlan, J; North, PC; Dubocovich, ML; Hagan, RM GR196429: a nonindolic agonist at high-affinity melatonin receptors. J Pharmacol Exp Ther 285: 1239-45 (1998)
- Wang, SY; Shi, XC; Laborda, P Indole-based melatonin analogues: Synthetic approaches and biological activity. Eur J Med Chem 185: (2020)
- Di Giacomo, B; Bedini, A; Spadoni, G; Tarzia, G; Fraschini, F; Pannacci, M; Lucini, V Synthesis and biological activity of new melatonin dimeric derivatives. Bioorg Med Chem 15: 4643-50 (2007)
- Hoashi, Y; Takai, T; Kosugi, Y; Nakashima, M; Nakayama, M; Hirai, K; Uchikawa, O; Koike, T Discovery of a Potent and Orally Bioavailable Melatonin Receptor Agonist. J Med Chem 64: 3059-3074 (2021)
- Copinga, S; Tepper, PG; Grol, CJ; Horn, AS; Dubocovich, ML 2-Amido-8-methoxytetralins: a series of nonindolic melatonin-like agents. J Med Chem 36: 2891-8 (1993)
- Zlotos, DP; Jockers, R; Cecon, E; Rivara, S; Witt-Enderby, PA MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J Med Chem 57: 3161-85 (2014)
- de la Fuente Revenga, M; Herrera-Arozamena, C; Fernández-Sáez, N; Barco, G; García-Orue, I; Sugden, D; Rivara, S; Rodríguez-Franco, MI New coumarin-based fluorescent melatonin ligands. Design, synthesis and pharmacological characterization. Eur J Med Chem 103: 370-3 (2015)
- Thireau, J; Marteaux, J; Delagrange, P; Lefoulon, F; Dufourny, L; Guillaumet, G; Suzenet, F Original Design of Fluorescent Ligands by Fusing BODIPY and Melatonin Neurohormone. ACS Med Chem Lett 5: 158-61 (2014)
- Mari, M; Elisi, GM; Bedini, A; Lucarini, S; Retini, M; Lucini, V; Scaglione, F; Vincenzi, F; Varani, K; Castelli, R; Mor, M; Rivara, S; Spadoni, G 2-Arylmelatonin analogues: Probing the 2-phenyl binding pocket of melatonin MT Eur J Med Chem 243: (2022)
- He, F; Chou, CJ; Scheiner, M; Poeta, E; Yuan Chen, N; Gunesch, S; Hoffmann, M; Sotriffer, C; Monti, B; Maurice, T; Decker, M Melatonin- and Ferulic Acid-Based HDAC6 Selective Inhibitors Exhibit Pronounced Immunomodulatory Effects J Med Chem 64: 3794-3812 (2021)
- Bolteau, R; Descamps, F; Ettaoussi, M; Caignard, DH; Delagrange, P; Melnyk, P; Yous, S Quinazoline and phthalazine derivatives as novel melatonin receptor ligands analogues of agomelatine. Eur J Med Chem 189: (2020)
- Li, PK; Chu, GH; Gillen, ML; Witt-Enderby, PA Synthesis and receptor binding studies of quinolinic derivatives as melatonin receptor ligands Bioorg Med Chem Lett 7: 2177-2180 (1997)
- Fukatsu, K; Uchikawa, O; Kawada, M; Yamano, T; Yamashita, M; Kato, K; Hirai, K; Hinuma, S; Miyamoto, M; Ohkawa, S Synthesis of a novel series of benzocycloalkene derivatives as melatonin receptor agonists. J Med Chem 45: 4212-21 (2002)
- Kloubert, S; Mathé-Allainmat, M; Andrieux, J; Sicsic, S; Langlois, M Synthesis of benzocycloalkane derivatives as new conformationally restricted ligands for melatonin receptors. Bioorg Med Chem Lett 8: 3325-30 (1999)
- Rivara, S; Scalvini, L; Lodola, A; Mor, M; Caignard, DH; Delagrange, P; Collina, S; Lucini, V; Scaglione, F; Furiassi, L; Mari, M; Lucarini, S; Bedini, A; Spadoni, G Tetrahydroquinoline Ring as a Versatile Bioisostere of Tetralin for Melatonin Receptor Ligands. J Med Chem 61: 3726-3737 (2018)
- Lucini, V; Pannacci, M; Scaglione, F; Fraschini, F; Rivara, S; Mor, M; Bordi, F; Plazzi, PV; Spadoni, G; Bedini, A; Piersanti, G; Diamantini, G; Tarzia, G Tricyclic alkylamides as melatonin receptor ligands with antagonist or inverse agonist activity. J Med Chem 47: 4202-12 (2004)
- Witt-Enderby, PA; Chu, GH; Gillen, ML; Li, PK Development of a high-affinity ligand that binds irreversibly to Mel1b melatonin receptors. J Med Chem 40: 4195-8 (1998)
- Sun, LQ; Chen, J; Bruce, M; Deskus, JA; Epperson, JR; Takaki, K; Johnson, G; Iben, L; Mahle, CD; Ryan, E; Xu, C Synthesis and structure-activity relationship of novel benzoxazole derivatives as melatonin receptor agonists. Bioorg Med Chem Lett 14: 3799-802 (2004)
- Uchikawa, O; Fukatsu, K; Tokunoh, R; Kawada, M; Matsumoto, K; Imai, Y; Hinuma, S; Kato, K; Nishikawa, H; Hirai, K; Miyamoto, M; Ohkawa, S Synthesis of a novel series of tricyclic indan derivatives as melatonin receptor agonists. J Med Chem 45: 4222-39 (2002)
- Feng, Y; Jiang, X; Liu, W; Lu, H The location, physiology, pathology of hippocampus Melatonin MT2 receptor and MT2-selective modulators. Eur J Med Chem 262:
- Faust, R; Garratt, PJ; Jones, R; Yeh, LK; Tsotinis, A; Panoussopoulou, M; Calogeropoulou, T; Teh, MT; Sugden, D Mapping the melatonin receptor. 6. Melatonin agonists and antagonists derived from 6H-isoindolo[2,1-a]indoles, 5,6-dihydroindolo[2,1-a]isoquinolines, and 6,7-dihydro-5H-benzo[c]azepino[2,1-a]indoles. J Med Chem 43: 1050-61 (2000)
- Tarzia, G; Diamantini, G; Di Giacomo, B; Spadoni, G; Esposti, D; Nonno, R; Lucini, V; Pannacci, M; Fraschini, F; Stankov, BM 1-(2-Alkanamidoethyl)-6-methoxyindole derivatives: a new class of potent indole melatonin analogues. J Med Chem 40: 2003-10 (1997)
- Reppert, SM; Weaver, DR; Ebisawa, T Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13: 1177-85 (1994)
- Somalo-Barranco, G; Serra, C; Lyons, D; Piggins, HD; Jockers, R; Llebaria, A Design and Validation of the First Family of Photo-Activatable Ligands for Melatonin Receptors. J Med Chem 65: 11229-11240 (2022)
- Nonno, R; Lucini, V; Pannacci, M; Mazzucchelli, C; Angeloni, D; Fraschini, F; Stankov, BM Pharmacological characterization of the human melatonin Mel1a receptor following stable transfection into NIH3T3 cells. Br J Pharmacol 124: 485-92 (1998)
- Chen, SY; Geng, CA; Ma, YB; Huang, XY; Yang, XT; Su, LH; He, XF; Li, TZ; Deng, ZT; Gao, Z; Zhang, XM; Chen, JJ Polybenzyls from Gastrodia elata, their agonistic effects on melatonin receptors and structure-activity relationships. Bioorg Med Chem 27: 3299-3306 (2019)
- Jellimann, C; Mathé-Allainmat, M; Andrieux, J; Kloubert, S; Boutin, JA; Nicolas, JP; Bennejean, C; Delagrange, P; Langlois, M Synthesis of phenalene and acenaphthene derivatives as new conformationally restricted ligands for melatonin receptors. J Med Chem 43: 4051-62 (2000)
- Ettaoussi, M; Péres, B; Klupsch, F; Delagrange, P; Boutin, JA; Renard, P; Caignard, DH; Chavatte, P; Berthelot, P; Lesieur, D; Yous, S Design and synthesis of benzofuranic derivatives as new ligands at the melatonin-binding site MT3. Bioorg Med Chem 16: 4954-62 (2008)
- Leclerc, V; Ettaoussi, M; Rami, M; Farce, A; Boutin, JA; Delagrange, P; Caignard, DH; Renard, P; Berthelot, P; Yous, S Design and synthesis of naphthalenic derivatives as new ligands at the melatonin binding site MT3. Eur J Med Chem 46: 1622-9 (2011)
- Hasan, M; Genovese, S; Fiorito, S; Epifano, F; Witt-Enderby, PA Oxyprenylated Phenylpropanoids Bind to MT1 Melatonin Receptors and Inhibit Breast Cancer Cell Proliferation and Migration. J Nat Prod 80: 3324-3329 (2017)
- Spadoni, G; Balsamini, C; Diamantini, G; Tontini, A; Tarzia, G; Mor, M; Rivara, S; Plazzi, PV; Nonno, R; Lucini, V; Pannacci, M; Fraschini, F; Stankov, BM 2-N-acylaminoalkylindoles: design and quantitative structure-activity relationship studies leading to MT2-selective melatonin antagonists. J Med Chem 44: 2900-12 (2001)
- Du, H; Wang, J; Zhang, X; Hu, Z A novel quantitative structure-activity relationship method to predict the affinities of MT3 melatonin binding site. Eur J Med Chem 43: 2861-9 (2008)
- Rivara, S; Lorenzi, S; Mor, M; Plazzi, PV; Spadoni, G; Bedini, A; Tarzia, G Analysis of structure-activity relationships for MT2 selective antagonists by melatonin MT1 and MT2 receptor models. J Med Chem 48: 4049-60 (2005)
- Castro-Palomino Laria, JC; Camacho Gómez, JA; Mendoza Lizaldez, A Derivatives of 2-aminopyridine as adenosine A2B receptor antagonists and ligands of the melatonin MT3 receptors US Patent US10253017 (2019)
- Jeanty, M; Suzenet, F; Delagrange, P; Nosjean, O; Boutin, JA; Caignard, DH; Guillaumet, G Design and synthesis of 1-(2-alkanamidoethyl)-6-methoxy-7-azaindole derivatives as potent melatonin agonists. Bioorg Med Chem Lett 21: 2316-9 (2011)
- Sun, LQ; Takaki, K; Chen, J; Iben, L; Knipe, JO; Pajor, L; Mahle, CD; Ryan, E; Xu, C N-[2-[2-(4-Phenylbutyl)benzofuran-4-yl]cyclopropylmethyl]acetamide: an orally bioavailable melatonin receptor agonist. Bioorg Med Chem Lett 14: 5157-60 (2004)
- Marot, C; Chavatte, P; Morin-Allory, L; Viaud, MC; Guillaumet, G; Renard, P; Lesieur, D; Michel, A Pharmacophoric search and 3D-QSAR comparative molecular field analysis studies on agonists of melatonin sheep receptors. J Med Chem 41: 4453-65 (1998)
- Li, TZ; Hu, J; Sun, JJ; Huang, XY; Geng, CA; Liu, SB; Zhang, XM; Chen, JJ Synthesis and biological evaluation of paeoveitol D derivatives as new melatonin receptor agonists with antidepressant activities. RSC Med Chem 13: 1212-1224 (2022)
- Sicsic, S; Serraz, I; Andrieux, J; Brémont, B; Mathé-Allainmat, M; Poncet, A; Shen, S; Langlois, M Three-dimensional quantitative structure-activity relationship of melatonin receptor ligands: a comparative molecular field analysis study. J Med Chem 40: 739-48 (1997)
- Faust, R; Garratt, PJ; Trujillo Pérez, MA; Piccio, VJ; Madsen, C; Stenstrøm, A; Frølund, B; Davidson, K; Teh, MT; Sugden, D 7-Substituted-melatonin and 7-substituted-1-methylmelatonin analogues: effect of substituents on potency and binding affinity. Bioorg Med Chem 15: 4543-51 (2007)
- El Kazzouli, S; Griffon du Bellay, A; Berteina-Raboin, S; Delagrange, P; Caignard, DH; Guillaumet, G Design and synthesis of 2-phenylimidazo[1,2-a]pyridines as a novel class of melatonin receptor ligands. Eur J Med Chem 46: 4252-7 (2011)
- Carocci, A; Catalano, A; Lovece, A; Lentini, G; Duranti, A; Lucini, V; Pannacci, M; Scaglione, F; Franchini, C Design, synthesis, and pharmacological effects of structurally simple ligands for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem 18: 6496-511 (2010)
- Garratt, PJ; Travard, S; Vonhoff, S; Tsotinis, A; Sugden, D Mapping the melatonin receptor. 4. Comparison of the binding affinities of a series of substituted phenylalkyl amides. J Med Chem 39: 1797-805 (1996)
- Rivara, S; Lodola, A; Mor, M; Bedini, A; Spadoni, G; Lucini, V; Pannacci, M; Fraschini, F; Scaglione, F; Sanchez, RO; Gobbi, G; Tarzia, G N-(substituted-anilinoethyl)amides: design, synthesis, and pharmacological characterization of a new class of melatonin receptor ligands. J Med Chem 50: 6618-26 (2007)
- Depreux, P; Lesieur, D; Mansour, HA; Morgan, P; Howell, HE; Renard, P; Caignard, DH; Pfeiffer, B; Delagrange, P; Guardiola, B Synthesis and structure-activity relationships of novel naphthalenic and bioisosteric related amidic derivatives as melatonin receptor ligands. J Med Chem 37: 3231-9 (1994)
- Wallez, V; Durieux-Poissonnier, S; Chavatte, P; Boutin, JA; Audinot, V; Nicolas, JP; Bennejean, C; Delagrange, P; Renard, P; Lesieur, D Synthesis and structure-affinity-activity relationships of novel benzofuran derivatives as MT(2) melatonin receptor selective ligands. J Med Chem 45: 2788-800 (2002)
- Mathé-Allainmat, M; Gaudy, F; Sicsic, S; Dangy-Caye, AL; Shen, S; Brémont, B; Benatalah, Z; Langlois, M; Renard, P; Delagrange, P Synthesis of 2-amido-2,3-dihydro-1H-phenalene derivatives as new conformationally restricted ligands for melatonin receptors. J Med Chem 39: 3089-95 (1996)
- Morellato, L; Lefas-Le Gall, M; Langlois, M; Caignard, DH; Renard, P; Delagrange, P; Mathé-Allainmat, M Synthesis of new N-(arylcyclopropyl)acetamides and N-(arylvinyl)acetamides as conformationally-restricted ligands for melatonin receptors. Bioorg Med Chem Lett 23: 430-4 (2012)
- Hu, Y; Ho, MK; Chan, KH; New, DC; Wong, YH Synthesis of substituted N-[3-(3-methoxyphenyl)propyl] amides as highly potent MT(2)-selective melatonin ligands. Bioorg Med Chem Lett 20: 2582-5 (2010)
- Spadoni, G; Balsamini, C; Bedini, A; Diamantini, G; Di Giacomo, B; Tontini, A; Tarzia, G; Mor, M; Plazzi, PV; Rivara, S; Nonno, R; Pannacci, M; Lucini, V; Fraschini, F; Stankov, BM 2-[N-Acylamino(C1-C3)alkyl]indoles as MT1 melatonin receptor partial agonists, antagonists, and putative inverse agonists. J Med Chem 41: 3624-34 (1998)
- Hu, Y; Zhu, J; Chan, KH; Wong, YH Development of substituted N-[3-(3-methoxylphenyl)propyl] amides as MT(2)-selective melatonin agonists: improving metabolic stability. Bioorg Med Chem 21: 547-52 (2012)
- Spadoni, G; Bedini, A; Lucarini, S; Mari, M; Caignard, DH; Boutin, JA; Delagrange, P; Lucini, V; Scaglione, F; Lodola, A; Zanardi, F; Pala, D; Mor, M; Rivara, S Highly Potent and Selective MT2 Melatonin Receptor Full Agonists from Conformational Analysis of 1-Benzyl-2-acylaminomethyl-tetrahydroquinolines. J Med Chem 58: 7512-25 (2015)
- Calamini, B; Santarsiero, BD; Boutin, JA; Mesecar, AD Kinetic, thermodynamic and X-ray structural insights into the interaction of melatonin and analogues with quinone reductase 2. Biochem J 413: 81-91 (2008)
- Nonno, R; Pannacci, M; Lucini, V; Angeloni, D; Fraschini, F; Stankov, BM Ligand efficacy and potency at recombinant human MT2 melatonin receptors: evidence for agonist activity of some mt1-antagonists. Br J Pharmacol 127: 1288-94 (1999)
- de la Fuente Revenga, M; Fernández-Sáez, N; Herrera-Arozamena, C; Morales-García, JA; Alonso-Gil, S; Pérez-Castillo, A; Caignard, DH; Rivara, S; Rodríguez-Franco, MI Novel N-Acetyl Bioisosteres of Melatonin: Melatonergic Receptor Pharmacology, Physicochemical Studies, and Phenotypic Assessment of Their Neurogenic Potential. J Med Chem 58: 4998-5014 (2015)
- Rodriguez-Franco, MI; Fernandez-Bachiller, MI; Perez, C; Hernandez-Ledesma, B; Bartolome, B Novel tacrine-melatonin hybrids as dual-acting drugs for Alzheimer disease, with improved acetylcholinesterase inhibitory and antioxidant properties. J Med Chem 49: 459-62 (2006)
- Spadoni, G; Stankov, B; Duranti, A; Biella, G; Lucini, V; Salvatori, A; Fraschini, F 2-Substituted 5-methoxy-N-acyltryptamines: synthesis, binding affinity for the melatonin receptor, and evaluation of the biological activity. J Med Chem 36: 4069-74 (1993)
- Luo, XT; Wang, CM; Liu, Y; Huang, ZG New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant, and neuroprotective properties as innovative drugs for Alzheimer's disease. Eur J Med Chem 103: 302-11 (2015)
- Herrera-Arozamena, C; Estrada-Valencia, M; Pérez, C; Lagartera, L; Morales-García, JA; Pérez-Castillo, A; Franco-Gonzalez, JF; Michalska, P; Duarte, P; León, R; López, MG; Mills, A; Gago, F; García-Yagüe, ÁJ; Fernández-Ginés, R; Cuadrado, A; Rodríguez-Franco, MI Tuning melatonin receptor subtype selectivity in oxadiazolone-based analogues: Discovery of QR2 ligands and NRF2 activators with neurogenic properties. Eur J Med Chem 190: (2020)
- Liu, P; Cheng, M; Guo, J; Cao, D; Luo, J; Wan, Y; Fang, Y; Jin, Y; Xie, SS; Liu, J Dual functional antioxidant and butyrylcholinesterase inhibitors for the treatment of Alzheimer's disease: Design, synthesis and evaluation of novel melatonin-alkylbenzylamine hybrids. Bioorg Med Chem 78: (2023)
- Wang, J; Wang, ZM; Li, XM; Li, F; Wu, JJ; Kong, LY; Wang, XB Synthesis and evaluation of multi-target-directed ligands for the treatment of Alzheimer's disease based on the fusion of donepezil and melatonin. Bioorg Med Chem 24: 4324-4338 (2016)
- Rivara, S; Mor, M; Silva, C; Zuliani, V; Vacondio, F; Spadoni, G; Bedini, A; Tarzia, G; Lucini, V; Pannacci, M; Fraschini, F; Plazzi, PV Three-dimensional quantitative structure-activity relationship studies on selected MT1 and MT2 melatonin receptor ligands: requirements for subtype selectivity and intrinsic activity modulation. J Med Chem 46: 1429-39 (2003)
- Spadoni, G; Bedini, A; Furiassi, L; Mari, M; Mor, M; Scalvini, L; Lodola, A; Ghidini, A; Lucini, V; Dugnani, S; Scaglione, F; Piomelli, D; Jung, KM; Supuran, CT; Lucarini, L; Durante, M; Sgambellone, S; Masini, E; Rivara, S Identification of Bivalent Ligands with Melatonin Receptor Agonist and Fatty Acid Amide Hydrolase (FAAH) Inhibitory Activity That Exhibit Ocular Hypotensive Effect in the Rabbit. J Med Chem 61: 7902-7916 (2018)
- López-Iglesias, B; Pérez, C; Morales-García, JA; Alonso-Gil, S; Pérez-Castillo, A; Romero, A; López, MG; Villarroya, M; Conde, S; Rodríguez-Franco, MI New melatonin-N,N-dibenzyl(N-methyl)amine hybrids: potent neurogenic agents with antioxidant, cholinergic, and neuroprotective properties as innovative drugs for Alzheimer's disease. J Med Chem 57: 3773-85 (2014)
- Spadoni, G; Bedini, A; Orlando, P; Lucarini, S; Tarzia, G; Mor, M; Rivara, S; Lucini, V; Pannacci, M; Scaglione, F Bivalent ligand approach on N-{2-[(3-methoxyphenyl)methylamino]ethyl}acetamide: synthesis, binding affinity and intrinsic activity for MT(1) and MT(2) melatonin receptors. Bioorg Med Chem 19: 4910-6 (2011)
- Tsotinis, A; Vlachou, M; Papahatjis, DP; Calogeropoulou, T; Nikas, SP; Garratt, PJ; Piccio, V; Vonhoff, S; Davidson, K; Teh, MT; Sugden, D Mapping the melatonin receptor. 7. Subtype selective ligands based on beta-substituted N-acyl-5-methoxytryptamines and beta-substituted N-acyl-5-methoxy-1-methyltryptamines. J Med Chem 49: 3509-19 (2006)
- Koike, T; Takai, T; Hoashi, Y; Nakayama, M; Kosugi, Y; Nakashima, M; Yoshikubo, S; Hirai, K; Uchikawa, O Synthesis of a novel series of tricyclic dihydrofuran derivatives: discovery of 8,9-dihydrofuro[3,2-c]pyrazolo[1,5-a]pyridines as melatonin receptor (MT1/MT2) ligands. J Med Chem 54: 4207-18 (2011)
- Millan, MJ; Gobert, A; Lejeune, F; Dekeyne, A; Newman-Tancredi, A; Pasteau, V; Rivet, JM; Cussac, D The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J Pharmacol Exp Ther 306: 954-64 (2003)
- Teh, MT; Sugden, D Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores. Naunyn Schmiedebergs Arch Pharmacol 358: 522-8 (1998)
- Dubocovich, ML; Masana, MI; Iacob, S; Sauri, DM Melatonin receptor antagonists that differentiate between the human Mel1a and Mel1b recombinant subtypes are used to assess the pharmacological profile of the rabbit retina ML1 presynaptic heteroreceptor. Naunyn Schmiedebergs Arch Pharmacol 355: 365-75 (1997)
- Benchekroun, M; Romero, A; Egea, J; León, R; Michalska, P; Buendía, I; Jimeno, ML; Jun, D; Janockova, J; Sepsova, V; Soukup, O; Bautista-Aguilera, OM; Refouvelet, B; Ouari, O; Marco-Contelles, J; Ismaili, L The Antioxidant Additive Approach for Alzheimer's Disease Therapy: New Ferulic (Lipoic) Acid Plus Melatonin Modified Tacrines as Cholinesterases Inhibitors, Direct Antioxidants, and Nuclear Factor (Erythroid-Derived 2)-Like 2 Activators. J Med Chem 59: 9967-9973 (2016)
- ChEMBL_104616 (CHEMBL715564) Binding affinity towards melatonin receptor, determined in chicken brain membranes using [2-125I]melatonin
- ChEMBL_104930 (CHEMBL714710) Binding affinity against ovine pars tuberalis melatonin receptor using 2-[125I]- melatonin radioligand binding assay
- ChEMBL_104755 (CHEMBL712438) Binding affinity towards melatonin receptor
- ChEMBL_104780 (CHEMBL874168) inhibitory concentration against Melatonin receptor
- ChEMBL_624959 (CHEMBL1107082) Displacement of [3H]melatonin from human melatonin MT1 receptor expressed in CHO cells after 60 mins by scintillation counting
- ChEMBL_624960 (CHEMBL1107083) Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting
- ChEBML_104949 Binding affinity towards human melatonin receptor type 1A
- ChEBML_105091 In vitro receptor binding at MT1 (Melatonin) receptor.
- ChEBML_105255 In vitro receptor binding at MT2 (Melatonin) receptor.
- ChEBML_105277 Binding affinity towards human melatonin receptor type 1B
- ChEMBL_105242 (CHEMBL712572) Binding affinity against melatonin receptor type 1A
- ChEMBL_305096 (CHEMBL832400) Inhibitory concentration against Melatonin receptor type 1A
- ChEMBL_305097 (CHEMBL832401) Inhibitory concentration against Melatonin receptor type 1B
- ChEMBL_644101 (CHEMBL1212000) Inhibition of human melatonin receptor type 1B
- Binding Assay Binding assay using melatonin receptors 1 or 2.
- ChEMBL_104619 (CHEMBL715567) Binding affinity towards melatonin receptor in chicken brain.
- ChEMBL_105110 (CHEMBL715953) Binding Affinity (pKi) towards Melatonin receptor type 1A
- ChEMBL_105255 (CHEMBL710591) In vitro receptor binding at MT2 (Melatonin) receptor.
- ChEMBL_104752 (CHEMBL712435) Binding affinity towards melatonin receptor in Chicken retinal membranes
- ChEMBL_104939 (CHEMBL714718) Inhibitory concentration against melatonin receptor by nonlinear fiting strategies
- ChEMBL_105111 (CHEMBL715954) Binding Affinity (pKi) towards human Melatonin receptor type 1A
- ChEMBL_105243 (CHEMBL712573) Binding affinity against human Melatonin receptor type 1A (MT1)
- ChEMBL_105272 (CHEMBL858415) Binding Affinity (pKi) towards human Melatonin receptor type 1B
- ChEMBL_105275 (CHEMBL718943) Binding affinity against human Melatonin receptor type 1B (MT2)
- ChEBML_105102 Binding affinity against human MT1 melatonin receptor expressed in NIH3T3 cells.
- ChEBML_105266 Binding affinity against human MT2 melatonin receptor expressed in NIH3T3 cells
- ChEMBL_104615 (CHEMBL715563) Inhibition of 2-[125I]- iodomelatonin from chicken brain melatonin receptors
- ChEMBL_104620 (CHEMBL715568) Binding affinity towards melatonin receptor in Chicken brain Experiment 1
- ChEMBL_104751 (CHEMBL715876) Binding affinity towards melatonin receptor in Chicken brain Experiment 2
- ChEMBL_104774 (CHEMBL714919) Agonist activity against melatonin receptor in the presence of iodomelatonin
- ChEMBL_104775 (CHEMBL714920) Agonist activity against melatonin receptor in the presence of iodomelatonin
- ChEMBL_1824682 (CHEMBL4324446) Displacement of 2-[125I]iodomelatonin from melatonin receptor (unknown origin)
- ChEMBL_1824703 (CHEMBL4324467) Displacement of 2-[125I]iodomelatonin from chick brain melatonin receptor
- ChEMBL_2238442 (CHEMBL5152338) Binding affinity to human melatonin MT1 assessed as inhibition constant
- ChEMBL_104614 (CHEMBL713592) Functional activity against melatonin receptor in lightening Xenopus laevis tadpole skin
- ChEMBL_104784 (CHEMBL714927) Binding affinity for Melatonin receptor using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104925 (CHEMBL714705) Binding affinity to melatonin receptor measured on ovine pars tuberalis membrane
- ChEMBL_104772 (CHEMBL714917) Agonist activity against melatonin receptor was tested in the absence of iodomelatonin
- ChEMBL_104773 (CHEMBL714918) Agonist activity against melatonin receptor was tested in the absence of iodomelatonin
- ChEMBL_2272288 Agonist activity at human melatonin MT1 receptor stably expressing in human HEK293 cells
- ChEMBL_2272289 Agonist activity at human melatonin MT2 receptor stably expressing in human HEK293 cells
- ChEMBL_744413 (CHEMBL1772367) Binding affinity to low affinity melatonin (MT3) site of quinone reductase 2
- ChEMBL_104764 (CHEMBL714909) Binding affinity against chicken brain melatonin receptors using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104924 (CHEMBL714704) Monophasic inhibitory concentration against melatonin receptor was measured on ovine pars tuberalis membrane.
- ChEMBL_304028 (CHEMBL840206) Binding affinity for human recombinant Melatonin receptor type 2 expressed in NIH3T3 cells
- ChEMBL_304031 (CHEMBL840209) Binding affinity for human recombinant Melatonin receptor type 1 expressed in NIH3T3 cells
- ChEMBL_744409 (CHEMBL1772363) Binding affinity to human low affinity melatonin (MT3) site of quinone reductase 2
- ChEBML_104948 Binding affinity against human Melatonin receptor type 1A by using 2-[125I]iodomelatonin as radioligand
- ChEBML_105103 Displacement of 2-[125I]iodomelatonin from human Melatonin receptor type 1A expressed in CHO cells
- ChEBML_105267 Compound was tested for binding affinity against human Melatonin receptor type 1B in CHO cells
- ChEBML_105276 Binding affinity against human Melatonin receptor type 1B by using 2-[125I]iodomelatonin as radioligand
- ChEBML_219514 Binding affinity measured against Chicken brain melatonin receptor by using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104759 (CHEMBL710991) Effect of compound on Melatonin receptor in chicken brain in the presence of MnCl2
- ChEMBL_514503 (CHEMBL973545) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor expressed in CHO cells
- ChEMBL_514504 (CHEMBL973546) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor expressed in CHO cells
- ChEMBL_544427 (CHEMBL1016924) Displacement of [125I]iodomelatonin from MT3/QR2 melatonin binding site expressed in CHO cells
- ChEMBL_104753 (CHEMBL712436) Binding affinity towards melatonin receptor using 2-[125I]iodomelatonin as radioligand in chick brain membranes
- ChEMBL_104779 (CHEMBL714924) Inhibitory activity against melatonin receptor of quail optica tecta with 200 pM 2-[125] iodomelatonin
- ChEMBL_104785 (CHEMBL714928) Inhibition of 2-[125I]iodomelatonin binding to melatonin receptor in quail brain as 1/Ka
- ChEMBL_105267 (CHEMBL713139) Compound was tested for binding affinity against human Melatonin receptor type 1B in CHO cells
- ChEMBL_219514 (CHEMBL824491) Binding affinity measured against Chicken brain melatonin receptor by using 2-[125I]iodomelatonin as radioligand
- ChEMBL_104609 (CHEMBL712996) Effective concentration against human MT2 (Melatonin) receptor stably expressed in NIH3T3 cells in adenylyl cyclase assay
- ChEMBL_104758 (CHEMBL710990) Effect of compound on Melatonin receptor in chicken brain in the presence of GTP-gamma-S
- ChEMBL_104942 (CHEMBL713109) Inhibition of 2-[125I]iodomelatonin binding to Melatonin receptor 3 (MT3) of Syrian hamster brain membrane
- ChEMBL_104950 (CHEMBL713259) Inhibition of 2-[125I]iodomelatonin binding to human Melatonin receptor type 1A expressed in HEK293 cells
- ChEMBL_105104 (CHEMBL715948) Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1A expressed in CHO cells
- ChEMBL_105269 (CHEMBL713141) Inhibition of the 2-[125I]- iodomelatonin binding to Melatonin receptor type 1B expressed in CHO cells
- ChEMBL_105404 (CHEMBL710823) Inhibition of 2-[125I]iodomelatonin binding to human Melatonin receptor type 1B expressed in HEK293 cells
- ChEMBL_303517 (CHEMBL839631) Binding affinity against Melatonin receptor type 1A stably expressed in NIH3T3 cells using 2-[125I]iodomelatonin
- ChEMBL_303518 (CHEMBL839632) Binding affinity against Melatonin receptor type 1B stably expressed in NIH3T3 cells using 2-[125I]iodomelatonin
- ChEMBL_512176 (CHEMBL968035) Agonist activity at CPR119 transfected in Xenopus dermal melanophore assessed as dispersion of melatonin-induced pigmentation
- ChEBML_105098 Binding affinity against human Melatonin receptor type 1A by displacement of [125I]iodomelatonin stably expressed in CHO cells
- ChEBML_105100 Binding of 2-[125I]iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A
- ChEBML_105262 Binding affinity against human Melatonin receptor type 1B by displacement of [125I]iodomelatonin stably expressed in CHO cells
- ChEBML_105264 Binding of 2-[125I]iodomelatonin to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B
- ChEMBL_104611 (CHEMBL713590) Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay.
- ChEMBL_104754 (CHEMBL712437) Binding affinity in chicken brain membranes by using [2-125I]melatonin in a competition radioligand binding assay
- ChEMBL_104756 (CHEMBL712439) Binding affinity towards melatonin receptor was determined using 2-[125I]iodomelatonin as radioligand in chick brain membranes
- ChEMBL_104757 (CHEMBL712440) Competitive binding by the displacement of 2-[125I]- Iodomelatonin binding from melatonin receptors in chicken brain membranes
- ChEMBL_104760 (CHEMBL710992) In vitro binding affinity against melatonin receptor using 2-[125I]iodomelatonin (0.05 nM) and chicken brain membranes
- ChEMBL_104776 (CHEMBL714921) Inhibitory activity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as labelled ligand
- ChEMBL_104777 (CHEMBL714922) Inhibitory activity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as radiolabeled ligand
- ChEMBL_104783 (CHEMBL714926) Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin as labelled ligand
- ChEMBL_104940 (CHEMBL714719) Ability to inhibit 2-[125I]iodomelatonin specific binding to melatonin receptor 3 (MT3) of Syrian hamster brain.
- ChEMBL_105108 (CHEMBL856174) Melatonin receptor type 1A binding affinity measured using 2-[125I]iodomelatonin on ovine pars tuberalis membrane homogenates.
- ChEMBL_1286910 (CHEMBL3110824) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor expressed in CHO cells after 120 mins
- ChEMBL_1286911 (CHEMBL3110825) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor expressed in CHO cells after 120 mins
- ChEMBL_1560583 (CHEMBL3777315) Displacement of 2-[125I]Iodomelatonin from human MT1 melatonin receptor expressed in HEK cells after 120 mins
- ChEMBL_1560584 (CHEMBL3777316) Displacement of 2-[125I]Iodomelatonin from human MT2 melatonin receptor expressed in HEK cells after 120 mins
- ChEMBL_304060 (CHEMBL839735) Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor MT2 expressed in NIH3T3 rat fibroblast cells
- ChEMBL_105109 (CHEMBL715952) Binding affinity for melatonin 1A receptor was measured using 2-[125I]iodomelatonin on ovine pars tuberalis membrane homogenates.
- ChEMBL_105244 (CHEMBL712574) Binding affinity for melatonin receptor type 1B, expressed in HEK293 cells (2-[125I]iodomelatonin is used as radioligand)
- ChEMBL_105273 (CHEMBL713144) Binding affinity towards melatonin receptor type 1B stably expressed in NIH3T3 rat fibroblast cells using 2-[125I]iodomelatonin
- ChEMBL_1499384 (CHEMBL3583713) Displacement of 2-[125I]-iodomelatonin from human melatonin receptor-1 transfected in CHO cell membranes after 120 mins
- ChEMBL_1499385 (CHEMBL3583714) Displacement of 2-[125I]-iodomelatonin from human melatonin receptor-2 transfected in CHO cell membranes after 120 mins
- ChEMBL_104613 (CHEMBL884504) The functional activity on melatonin receptor was evaluated by its potency to lighten the skin of Xenopus laevis tadpoles
- ChEMBL_104782 (CHEMBL714925) Binding affinity against melatonin receptor in the quail optica tecta using 2-[125] iodomelatonin (100 pM) as labelled ligand
- ChEMBL_104944 (CHEMBL713111) Binding affinity for human melatonin receptor type 1A, expressed in HEK293 cells (2-[125I]iodomelatonin is used as radioligand)
- ChEMBL_104946 (CHEMBL713113) Ability to inhibit 2-[125I]iodomelatonin specific binding to human melatonin receptor type 1A (MT1) expressed in CHO cells.
- ChEMBL_105080 (CHEMBL711325) Agonist potency determined by [35S]GTP gamma-S binding assay using CHO cell lines for Melatonin receptor type 1A
- ChEMBL_105248 (CHEMBL709923) Agonist potency determined by [35S]GTP gamma-S binding assay using CHO cell lines for Melatonin receptor type 1B
- ChEMBL_1499387 (CHEMBL3583716) Agonist activity at human melatonin receptor-1 transfected in CHO cell membranes after 1 hr by GTPgammaS binding assay
- ChEMBL_1499389 (CHEMBL3583718) Agonist activity at human melatonin receptor-2 transfected in CHO cell membranes after 1 hr by GTPgammaS binding assay
- ChEMBL_303582 (CHEMBL828981) Inhibition of 2-[125I]iodomelatonin binding to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1A
- ChEMBL_303583 (CHEMBL828124) Inhibition of 2-[125I]iodomelatonin binding to membrane preparations of NIH3T3 cells stably expressing human Melatonin receptor type 1B
- ChEMBL_304083 (CHEMBL838521) Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor type 1A (MT1) expressed in NIH3T3 rat fibroblast cells
- ChEMBL_304084 (CHEMBL838522) Inhibition of 2-[125I]iodomelatonin binding to human melatonin receptor type 1B (MT2) expressed in NIH3T3 rat fibroblast cells
- ChEMBL_310710 (CHEMBL838055) Agonist activity towards human Melatonin receptor type 1A was determined by its ability to inhibit forskolin stimulated cAMP accumulation
- ChEMBL_310711 (CHEMBL838056) Agonist activity towards human Melatonin receptor type 1B was determined by its ability to inhibit forskolin stimulated cAMP accumulation
- ChEMBL_939502 (CHEMBL2328137) Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method
- ChEMBL_939503 (CHEMBL2328138) Displacement of [3H]melatonin from human MT1 receptor expressed in CHO cells after 60 mins by microbeta scintillation method
- ChEMBL_105083 (CHEMBL710854) Intrinsic activity of Melatonin receptor type 1A evaluated on [35S]GTP-gamma-S, binding in Chinese hamster ovarian (CHO) cells
- ChEMBL_104778 (CHEMBL714923) Inhibitory activity against melatonin receptor of quail optica tecta with 200 pM 2-[125] iodomelatonin as gamma-S (10e-4 M)
- ChEMBL_105082 (CHEMBL710853) Intrinsic activity evaluated on [35S]GTP -gamma-S binding in hamster ovarian (CHO) cells, stably expressing human Melatonin receptor type 1A
- ChEMBL_105101 (CHEMBL715945) Binding affinity towards recombinant human melatonin receptor type 1A expressed in NIH 3T3 cells using 2-[121I]iodomelatonin radioligand binding assay
- ChEMBL_105249 (CHEMBL709924) Intrinsic activity at human Melatonin receptor type 1B evaluated on [35S]GTP-gamma-S, binding in Chinese hamster ovarian (CHO) cells
- ChEMBL_105250 (CHEMBL710586) Intrinsic activity at human Melatonin receptor type 1B evaluated on [35S]GTP-gamma-S, binding in Chinese hamster ovarian (CHO) cells
- ChEMBL_105265 (CHEMBL872558) Binding affinity towards recombinant human melatonin receptor type 1B expressed in NIH 3T3 cells using 2-[121I]iodomelatonin radioligand binding assay
- ChEMBL_1499416 (CHEMBL3583851) Agonist activity at human recombinant melatonin receptor-1 expressed in CHO cells assessed as effect on impedance by cellular dielectric spectroscopy
- ChEMBL_105081 (CHEMBL711326) Intrinsic activity evaluated on [35S]GTP -gamma-S binding in Chinese hamster ovarian (CHO) cells, stably expressing human Melatonin receptor type 1A
- ChEMBL_105099 (CHEMBL714106) Binding affinity on human melatonin receptor type 1A stably transfected in human embryonic kidney (HEK 293) using 2-[125I]iodomelatonin as radioligand.
- ChEMBL_105112 (CHEMBL715955) Binding affinity towards melatonin receptor type 1A stably expressed in NIH3T3 rat fibroblast cells using 2-[125I]iodomelatonin (100 pM) as radioligand
- ChEMBL_105097 (CHEMBL714104) Binding affinity for human Melatonin receptor type 1A stably transfected in human embryonic kidney cells (HEK 293) using 2-[125I]iodomelatonin as radioligand
- ChEMBL_105261 (CHEMBL710751) Binding affinity for human Melatonin receptor type 1B stably transfected in human embryonic kidney cells (HEK 293) using 2-[125I]iodomelatonin as radioligand
- ChEMBL_105263 (CHEMBL715855) Binding affinity on human melatonin receptor type 1B stably transfected in human embryonic kidney (HEK 293) cells using 2-[125I]iodomelatonin as radioligand.
- ChEMBL_2026951 (CHEMBL4681109) Agonist activity at MT1 (unknown origin) expressed in HEK293T cells incubated for 15 mins by melatonin Gi/o-mediated cAMP inhibition GloSensor assay
- ChEMBL_2026952 (CHEMBL4681110) Agonist activity at MT2(unknown origin) expressed in HEK293T cells incubated for 15 mins by melatonin Gi/o-mediated cAMP inhibition GloSensor assay
- ChEMBL_1499414 (CHEMBL3583849) Agonist activity at human recombinant melatonin receptor-2 expressed in CHO cells assessed as decrease of cAMP level after 10 mins by HTRF assay
- ChEMBL_2228632 (CHEMBL5142145) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor stably expressing in human HEK293 cell membranes under dark condition by radioligand based competition binding assay
- ChEMBL_2228635 (CHEMBL5142148) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor stably expressing in human HEK293 cell membranes under dark condition by radioligand based competition binding assay
- Binding to Melatonin MT3 Binding Sites The experiment of binding to MT3 sites was carried out on hamster brain membranes using [125I]2-iodomelatonin as radioligand in accordance with the protocol described by Pickering, D. S et al. (Pickering, D. S et al, 1990, Pharmacological characterization of melatonin binding sites in Syrian hamster hypothalamus, Eur J Pharmacol. 1990 Jan. 3; 175(1):71-7).
- ChEMBL_2228633 (CHEMBL5142146) Displacement of 2-[125I]iodomelatonin from human melatonin MT1 receptor stably expressing in human HEK293 cell membranes in presence of light-activated compounds by radioligand based competition binding assay
- ChEMBL_2228636 (CHEMBL5142149) Displacement of 2-[125I]iodomelatonin from human melatonin MT2 receptor stably expressing in human HEK293 cell membranes in presence of light-activated compounds by radioligand based competition binding assay
- ChEMBL_2228637 (CHEMBL5142150) Agonist activity at human melatonin MT1 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins under dark condition by cAMP assay
- ChEMBL_2228641 (CHEMBL5142154) Agonist activity at human melatonin MT2 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins under dark condition by cAMP assay
- ChEMBL_2228638 (CHEMBL5142151) Agonist activity at human melatonin MT1 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins in presence of light-activated compounds by cAMP assay
- ChEMBL_2228642 (CHEMBL5142155) Agonist activity at human melatonin MT2 receptor stably expressing in human HEK293 cells assessed as inhibition of forskolin-stimulated cAMP accumulation after 30 mins in presence of light-activated compounds by cAMP assay
 BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy
BDBM82509 melatonin, 6-Hydroxy Melatonin,6-Hydroxy BDBM85063 Melatonin,6-Cl
BDBM85063 Melatonin,6-Cl Melatonin,2-Chloro BDBM85236
Melatonin,2-Chloro BDBM85236 melatonin, 6-Methoxy BDBM82556
melatonin, 6-Methoxy BDBM82556 BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo
BDBM29611 CHEMBL289233 2-Iodomelatonin Melatonin,2-Iodo CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin
CHEMBL498494 BDBM50272623 (+/-)-2-(2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me
BDBM85061 CAS_73-31-4 Melatonin,6-Cl-2-Me CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622
CHEMBL498493 2-((S)-2-Hydroxymethylindolin-1-ylmethyl)-melatonin BDBM50272622 BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin
BDBM50272621 CHEMBL525374 (+/-)-2-(2-Hydroxymethyl-5-methoxyindolin-1-ylmethyl)-melatonin BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4
BDBM85055 Melatonin,6,7 di-cl-2-Me CAS_73-31-4 BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide
BDBM9019 CHEMBL45 Melatonin N-[2-(5-methoxy-1H-indol-3-yl)ethyl]acetamide BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy
BDBM50066958 N-[2-(6-Methoxy-1H-indol-3-yl)-ethyl]-acetamide CHEMBL33099 Melatonin,6-Methoxy Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110
Melatonin,2-Phenyl N-[2-(5-Methoxy-2-phenyl-1H-indol-3-yl)-ethyl]-acetamide CHEMBL15060 BDBM50034110 CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin)
CHEMBL33415 Melatonin,2-Bromo N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043287 N-[2-(2-Bromo-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(2-Bromomelatonin) N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730
N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide(6-Chloromelatonin) melatonin, 6-Chloro N-[2-(6-Chloro-5-methoxy-1H-indol-3-yl)-ethyl]-acetamide BDBM50043289 CHEMBL34730 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9006 N-[2-(1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide Tacrine-Melatonin Hybrid 3a BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide
BDBM9007 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 3b N-[2-(1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide
6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-hydroxy-1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 11a BDBM9018 N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014
N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-7-(1,2,3,4-tetrahydroacridin-9-ylamino)heptanamide 7-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-5-metoxy-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 7b BDBM9014 Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide
Tacrine-Melatonin Hybrid 7a BDBM9013 N-[2-(5-methoxy-1H-indol-3-yl)ethyl]-6-(1,2,3,4-tetrahydroacridin-9-ylamino)hexanamide 6-(1,2,3,4-Tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a
6-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9011 6-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 6a BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a
BDBM9008 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 4a BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide
BDBM9009 7-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide Tacrine-Melatonin Hybrid 4b 7-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010
Tacrine-Melatonin Hybrid 5a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]hexanamide BDBM9010 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide
7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(1H-indol-3-yl)-ethyl]-amide BDBM9012 Tacrine-Melatonin Hybrid 6b CHEMBL199585 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(1H-indol-3-yl)ethyl]heptanamide BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide
BDBM9017 Tacrine-Melatonin Hybrid 10b 7-[(6,8-dichloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]heptanamide 7-(6,8-Dichloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-heptanoic acid [2-(5-methoxy-1Hindol-3-yl)-ethyl]-amide 6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a
6-(6-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide BDBM9015 6-[(6-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide Tacrine-Melatonin Hybrid 8a BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide
BDBM9016 Tacrine-Melatonin Hybrid 9a 6-(8-Chloro-1,2,3,4-tetrahydro-acridin-9-ylamino)-hexanoic acid [2-(5-methoxy-1H-indol-3-yl)-ethyl]-amide 6-[(8-chloro-1,2,3,4-tetrahydroacridin-9-yl)amino]-N-[2-(5-methoxy-1H-indol-3-yl)ethyl]hexanamide