 US9187470, 104 (SB203580) BDBM192501 US10865384, Compound SB203580
US9187470, 104 (SB203580) BDBM192501 US10865384, Compound SB203580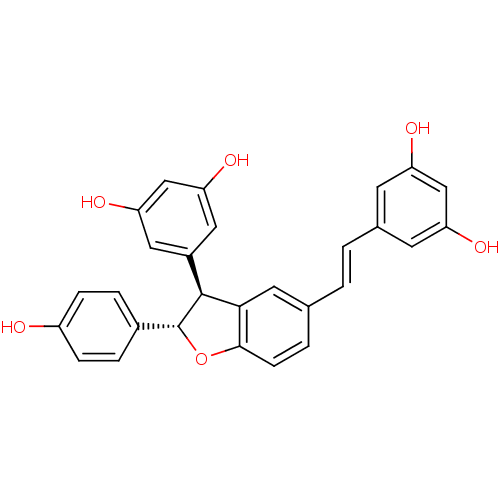 CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer
CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer Azo-Resveratrol CHEMBL2208146 BDBM50486146
Azo-Resveratrol CHEMBL2208146 BDBM50486146 CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate
CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate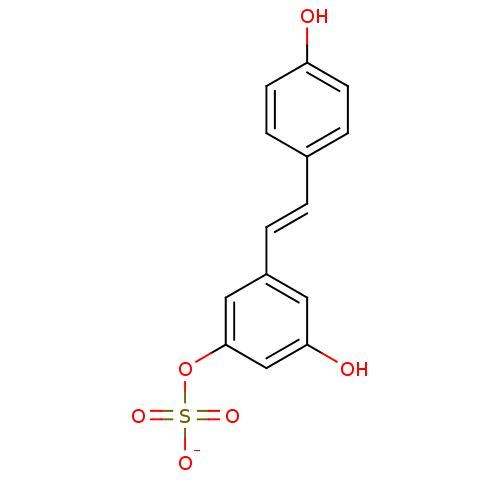 Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816
Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816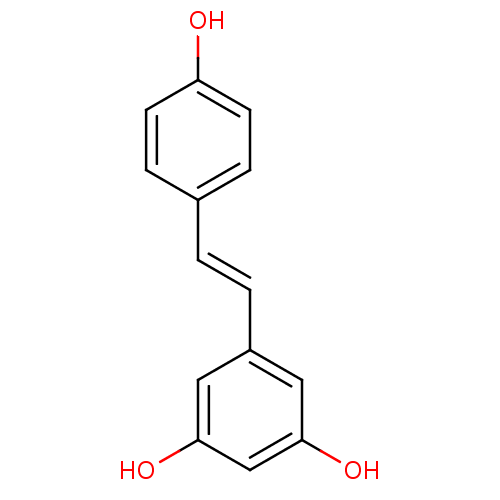 BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f
BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f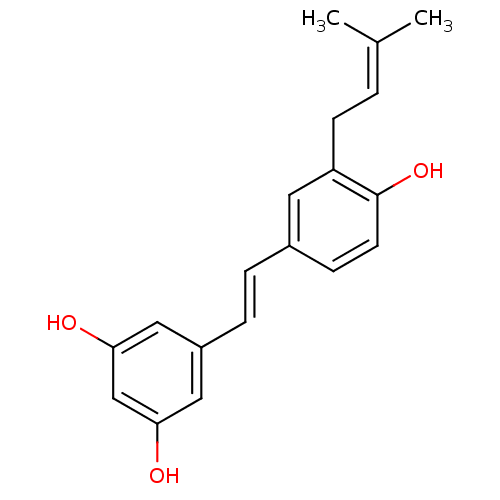 3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145
3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145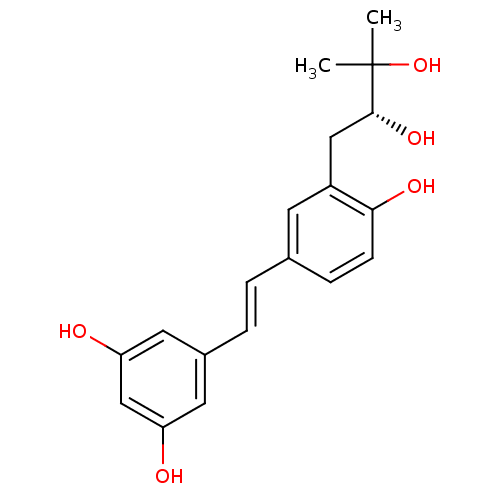 BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol
BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol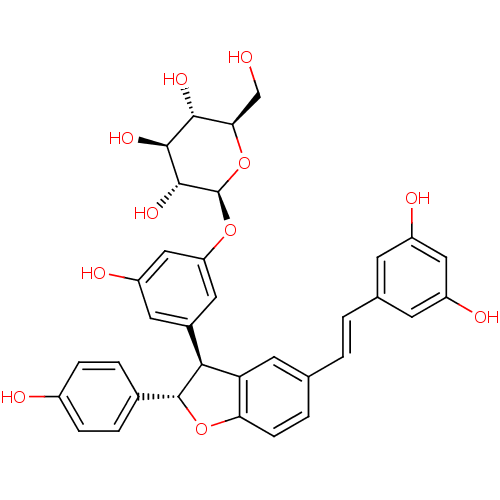 CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812
CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812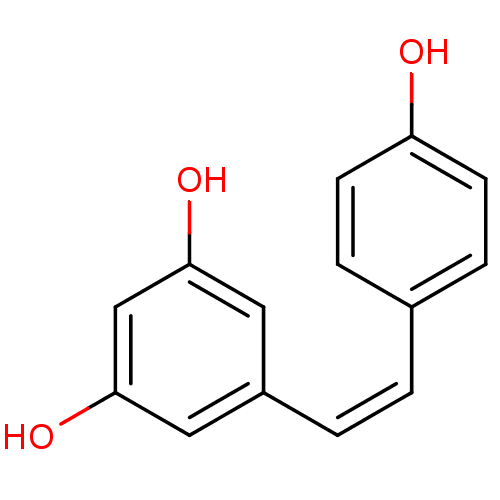 5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol
5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol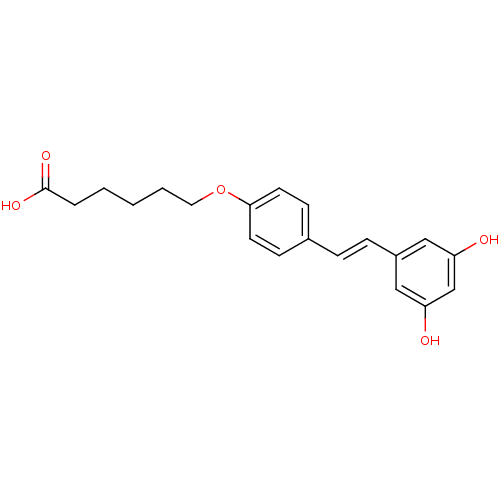 Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid
Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid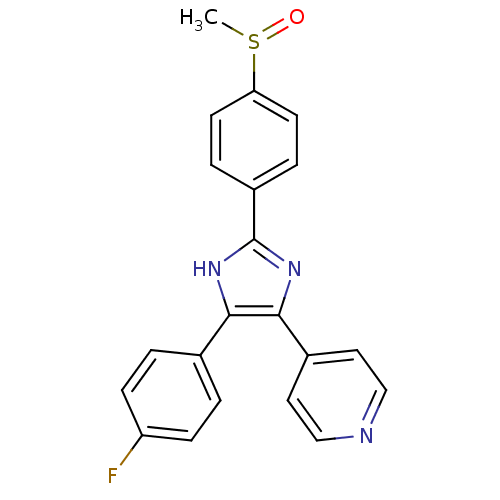 4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580
4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580
- Yokoyama, T; Kusaka, K; Mizuguchi, M; Nabeshima, Y; Fujiwara, S Resveratrol Derivatives Inhibit Transthyretin Fibrillization: Structural Insights into the Interactions between Resveratrol Derivatives and Transthyretin. J Med Chem 66: 15511-15523 (2023)
- Grau, L; Soucek, R; Pujol, MD Resveratrol derivatives: Synthesis and their biological activities. Eur J Med Chem 246: (2023)
- Cichewicz, RH; Kouzi, SA; Hamann, MT Dimerization of resveratrol by the grapevine pathogen Botrytis cinerea. J Nat Prod 63: 29-33 (2000)
- Mayhoub, AS; Marler, L; Kondratyuk, TP; Park, EJ; Pezzuto, JM; Cushman, M Optimizing thiadiazole analogues of resveratrol versus three chemopreventive targets. Bioorg Med Chem 20: 510-20 (2011)
- Nalli, M; Ortar, G; Moriello, AS; Morera, E; Di Marzo, V; De Petrocellis, L TRPA1 channels as targets for resveratrol and related stilbenoids. Bioorg Med Chem Lett 26: 899-902 (2016)
- Low, JL; Jürjens, G; Seayad, J; Seow, J; Ting, S; Laco, F; Reuveny, S; Oh, S; Chai, CL Tri-substituted imidazole analogues of SB203580 as inducers for cardiomyogenesis of human embryonic stem cells. Bioorg Med Chem Lett 23: 3300-3 (2013)
- Pettit, GR; Grealish, MP; Jung, MK; Hamel, E; Pettit, RK; Chapuis, JC; Schmidt, JM Antineoplastic agents. 465. Structural modification of resveratrol: sodium resverastatin phosphate. J Med Chem 45: 2534-42 (2002)
- Cardullo, N; Spatafora, C; Musso, N; Barresi, V; Condorelli, D; Tringali, C Resveratrol-Related Polymethoxystilbene Glycosides: Synthesis, Antiproliferative Activity, and Glycosidase Inhibition. J Nat Prod 78: 2675-83 (2015)
- Fantacuzzi, M; Amoroso, R; Carradori, S; De Filippis, B Resveratrol-based compounds and neurodegeneration: Recent insight in multitarget therapy. Eur J Med Chem 233: (2022)
- Hoshino, J; Park, EJ; Kondratyuk, TP; Marler, L; Pezzuto, JM; van Breemen, RB; Mo, S; Li, Y; Cushman, M Selective synthesis and biological evaluation of sulfate-conjugated resveratrol metabolites. J Med Chem 53: 5033-43 (2010)
- Das, J; Pany, S; Majhi, A Chemical modifications of resveratrol for improved protein kinase C alpha activity. Bioorg Med Chem 19: 5321-33 (2011)
- Mayhoub, AS; Marler, L; Kondratyuk, TP; Park, EJ; Pezzuto, JM; Cushman, M Optimization of the aromatase inhibitory activities of pyridylthiazole analogues of resveratrol. Bioorg Med Chem 20: 2427-34 (2012)
- Cheng, G; Xu, P; Zhang, M; Chen, J; Sheng, R; Ma, Y Resveratrol-maltol hybrids as multi-target-directed agents for Alzheimer's disease. Bioorg Med Chem 26: 5759-5765 (2018)
- Xu, P; Zhang, M; Sheng, R; Ma, Y Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ Eur J Med Chem 127: 174-186 (2017)
- Tang, YW; Shi, CJ; Yang, HL; Cai, P; Liu, QH; Yang, XL; Kong, LY; Wang, XB Synthesis and evaluation of isoprenylation-resveratrol dimer derivatives against Alzheimer's disease. Eur J Med Chem 163: 307-319 (2019)
- Aldawsari, FS; Aguiar, RP; Wiirzler, LA; Aguayo-Ortiz, R; Aljuhani, N; Cuman, RK; Medina-Franco, JL; Siraki, AG; Velázquez-Martínez, CA Anti-inflammatory and antioxidant properties of a novel resveratrol-salicylate hybrid analog. Bioorg Med Chem Lett 26: 1411-5 (2016)
- Vilar, S; Quezada, E; Santana, L; Uriarte, E; Yánez, M; Fraiz, N; Alcaide, C; Cano, E; Orallo, F Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids. Bioorg Med Chem Lett 16: 257-61 (2005)
- Jerábek, J; Uliassi, E; Guidotti, L; Korábecný, J; Soukup, O; Sepsova, V; Hrabinova, M; Kuca, K; Bartolini, M; Peña-Altamira, LE; Petralla, S; Monti, B; Roberti, M; Bolognesi, ML Tacrine-resveratrol fused hybrids as multi-target-directed ligands against Alzheimer's disease. Eur J Med Chem 127: 250-262 (2017)
- Ahmadi, R; Ebrahimzadeh, MA Resveratrol - A comprehensive review of recent advances in anticancer drug design and development. Eur J Med Chem 200: (2020)
- Jiang, YL Design, synthesis and spectroscopic studies of resveratrol aliphatic acid ligands of human serum albumin. Bioorg Med Chem 16: 6406-14 (2008)
- Duan, YC; Guan, YY; Zhai, XY; Ding, LN; Qin, WP; Shen, DD; Liu, XQ; Sun, XD; Zheng, YC; Liu, HM Discovery of resveratrol derivatives as novel LSD1 inhibitors: Design, synthesis and their biological evaluation. Eur J Med Chem 126: 246-258 (2017)
- Menezes, JCJMDS; Diederich, MF Natural dimers of coumarin, chalcones, and resveratrol and the link between structure and pharmacology. Eur J Med Chem 182: (2019)
- Song, YM; Ha, YM; Kim, JA; Chung, KW; Uehara, Y; Lee, KJ; Chun, P; Byun, Y; Chung, HY; Moon, HR Synthesis of novel azo-resveratrol, azo-oxyresveratrol and their derivatives as potent tyrosinase inhibitors. Bioorg Med Chem Lett 22: 7451-5 (2012)
- Nakao, S; Mabuchi, M; Wang, S; Kogure, Y; Shimizu, T; Noguchi, K; Tanaka, A; Dai, Y Synthesis of resveratrol derivatives as new analgesic drugs through desensitization of the TRPA1 receptor. Bioorg Med Chem Lett 27: 3167-3172 (2017)
- Hao, XD; Chang, J; Qin, BY; Zhong, C; Chu, ZB; Huang, J; Zhou, WJ; Sun, X Synthesis, estrogenic activity, and anti-osteoporosis effects in ovariectomized rats of resveratrol oligomer derivatives. Eur J Med Chem 102: 26-38 (2015)
- Lu, C; Guo, Y; Yan, J; Luo, Z; Luo, HB; Yan, M; Huang, L; Li, X Design, synthesis, and evaluation of multitarget-directed resveratrol derivatives for the treatment of Alzheimer's disease. J Med Chem 56: 5843-59 (2014)
- Mayhoub, AS; Marler, L; Kondratyuk, TP; Park, EJ; Pezzuto, JM; Cushman, M Optimization of thiazole analogues of resveratrol for induction of NAD(P)H:quinone reductase 1 (QR1). Bioorg Med Chem 20: 7030-9 (2012)
- Yao, RS; Lu, XQ; Guan, QX; Zheng, L; Lu, X; Ruan, BF Synthesis and biological evaluation of some novel resveratrol amide derivatives as potential anti-tumor agents. Eur J Med Chem 62: 222-31 (2013)
- Lee, I; Choe, YS; Choi, JY; Lee, KH; Kim, BT Synthesis and evaluation of ¹⁸F-labeled styryltriazole and resveratrol derivatives for β-amyloid plaque imaging. J Med Chem 55: 883-92 (2012)
- Samaradivakara, SP; Samarasekera, R; Handunnetti, SM; Weerasena, OVDSJ; Al-Hamashi, AA; Slama, JT; Taylor, WR; Alhadidi, Q; Shah, ZA; Perera, L; Tillekeratne, LMV A Bioactive Resveratrol Trimer from the Stem Bark of the Sri Lankan Endemic Plant Vateria copallifera. J Nat Prod 81: 1693-1700 (2018)
- St John, SE; Jensen, KC; Kang, S; Chen, Y; Calamini, B; Mesecar, AD; Lipton, MA Design, synthesis, biological and structural evaluation of functionalized resveratrol analogues as inhibitors of quinone reductase 2. Bioorg Med Chem 21: 6022-37 (2013)
- Herrera-Arozamena, C; Estrada-Valencia, M; López-Caballero, P; Pérez, C; Morales-García, JA; Pérez-Castillo, A; Sastre, ED; Fernández-Mendívil, C; Duarte, P; Michalska, P; Lombardía, J; Senar, S; León, R; López, MG; Rodríguez-Franco, MI Resveratrol-Based MTDLs to Stimulate Defensive and Regenerative Pathways and Block Early Events in Neurodegenerative Cascades. J Med Chem 65: 4727-4751 (2022)
- de Medina, P; Casper, R; Savouret, JF; Poirot, M Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem 48: 287-91 (2005)
- Resveratrol-derived inhibitors of the E3 ubiquitin ligase PELI1 inhibit the metastasis of triple-negative breast cancer.
- Ruan, BF; Cheng, HJ; Ren, J; Li, HL; Guo, LL; Zhang, XX; Liao, C Novel 2H-chromen-2-one derivatives of resveratrol: Design, synthesis, modeling and use as human monoamine oxidase inhibitors. Eur J Med Chem 103: 185-90 (2015)
- Sun, B; Hoshino, J; Jermihov, K; Marler, L; Pezzuto, JM; Mesecar, AD; Cushman, M Design, synthesis, and biological evaluation of resveratrol analogues as aromatase and quinone reductase 2 inhibitors for chemoprevention of cancer. Bioorg Med Chem 18: 5352-66 (2010)
- Mikstacka, R; Rimando, AM; Dutkiewicz, Z; Stefanski, T; Sobiak, S Design, synthesis and evaluation of the inhibitory selectivity of novel trans-resveratrol analogues on human recombinant CYP1A1, CYP1A2 and CYP1B1. Bioorg Med Chem 20: 5117-26 (2012)
- Kang, SS; Cuendet, M; Endringer, DC; Croy, VL; Pezzuto, JM; Lipton, MA Synthesis and biological evaluation of a library of resveratrol analogues as inhibitors of COX-1, COX-2 and NF-kappaB. Bioorg Med Chem 17: 1044-54 (2009)
- Yang, X; Qiang, X; Li, Y; Luo, L; Xu, R; Zheng, Y; Cao, Z; Tan, Z; Deng, Y Pyridoxine-resveratrol hybrids Mannich base derivatives as novel dual inhibitors of AChE and MAO-B with antioxidant and metal-chelating properties for the treatment of Alzheimer's disease. Bioorg Chem 71: 305-314 (2017)
- LanthaScreen Eu Kinase Binding Assay Table 3: Small molecules were tested for their inhibitory activities towards casein kinase 1 delta (CK1δ) and 1 epsilon (CK1ϵ). The in vitro LanthaScreen Eu kinase binding assay was used to determine the IC50 values for 15 of the synthesized compounds and 5 commercial inhibitors including SB203580 (Table 3). Tested compounds displayed a wide range of affinity between 6.8 nM (high affinity=strong inhibition) to values over 1000 nM (low affinity=very weak inhibition).
- Inhibition Assay The actual target(s) whereby the most active substituted trans-stilbenes inhibit the TNFα-induced activation of NF-κB remains to be identified. Resveratrol has been shown to suppress the TNF-induced phosphorylation and nuclear translocation of the p65 subunit of NF-κB.38 Both IKKα and IKKβ are able to catalyze the phosphorylation of p65, although through different signaling pathways,39 and are potential targets. Likewise, one or more of the kinases that activate IKK by phosphorylation, in response to TNFα or to the numerous other activators of NF-κB,35 may be the targets.
- Kinase Assay Table 2: The p38 MAPK IC50 values of selected compounds except IM-32 to IM-44 were determined using the HitHunter p38 MAP kinase binding assay from DiscoveRx Corporation (Fremont, Calif., USA). The p38 MAPK IC50 values of IM-32 to IM-44 and the CK1 IC50 values of selected compounds were determined using LanthaScreen Eu kinase binding assay from Invitrogen (Life Technologies, Carlsbad, Calif., USA). The assays were performed following the manufacturers' protocols in white 384-well plates (Cat. No. 3572; Corning Incorporated, Corning, N.Y., USA). SB203580 was used as the control in all assays. For the HitHunter p38 MAP kinase binding assay the compounds were dissolved in DMSO (5 mM stocks) and diluted to a final concentration of 2% (vol/vol) DMSO for all assays. Recombinant GST-tagged active p38α MAP kinase enzyme (Millipore, Billerica, Mass., USA) was used for the HitHunter p38 MAP kinase binding assay. For the LanthaScreen Eu kinase binding assay the compounds were dissolved in DMSO (5 mM stocks) and diluted to a final concentration of 1% (vol/vol) DMSO for all assays. Each data point was done in triplicate. The assay was run using JANUS Automated Workstation according to the protocol developed using WinPREP software (Perkin Elmer Inc., Waltham, Mass., USA). Tecan Infinite M1000 microplate reader (Tecan Group Ltd., Minnedorf, Switzerland) was used for luminescence measurements (HitHunter p38 MAP kinase binding assay) and fluorescence measurements (LanthaScreen Eu kinase binding assay; ex=340 nm, em=665, 615 nm). All data analysis was performed using GraphPad Prism 5 software (GraphPad Software Inc.). Inhibition curves and IC50 values were generated by nonlinear regression analysis and data represent mean±SEM.
 US9187470, 104 (SB203580) BDBM192501 US10865384, Compound SB203580
US9187470, 104 (SB203580) BDBM192501 US10865384, Compound SB203580 CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer
CHEMBL518935 resveratrol (E)-dehydrodimer BDBM50259651 resveratrol trans-dehydrodimer Azo-Resveratrol CHEMBL2208146 BDBM50486146
Azo-Resveratrol CHEMBL2208146 BDBM50486146 CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate
CHEMBL1173450 BDBM50322614 Resveratrol Potassium4,-Sulfate Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816
Resveratrol Potassium3-Sulfate BDBM50322615 CHEMBL1173685 CHEMBL1823816 BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f
BDBM23926 (E)-resveratrol cid_445154 US20240398793, Compound 1 Resveratol 5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol trans-resveratrol resveratrol US11866416, Example 7 CHEMBL165 Stilbene, 2f 3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145
3-(gamma,gamma-dimethylallyl)resveratrol BDBM50269596 CHEMBL457145 BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol
BDBM50269597 CHEMBL446319 3-(2,3-dihydroxy-3-methylbutyl)resveratrol CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812
CHEMBL503412 resveratrol (E)-dehydrodimer 11-O-beta-D-glucopyranoside BDBM50269812 5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol
5-[(1Z)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol 5-[(Z)-2-(4-hydroxyphenyl)vinyl]benzene-1,3-diol BDBM50131698 CHEMBL87333 US20240398793, Compound 2 cis-resveratrol (Z)-resveratrol Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid
Resveratrol hexanoic acid CHEMBL504510 BDBM50271238 6-{4-[(1E)-2-(3,5-dihydroxyphenyl)ethenyl]phenoxy}hexanoic acid 4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580
4-{4-(4-fluorophenyl)-2-[4-(methylsulfinyl)phenyl]-1H-imidazol-5-yl}pyridine SB-203580 4-[4-(4-fluorophenyl)-2-(4-methanesulfinylphenyl)-1H-imidazol-5-yl]pyridine BDBM13336 cid_176155 4-[4-(4-fluorophenyl)-2-(4-methylsulfinylphenyl)-1H-imidazol-5-yl]pyridine CHEMBL10 SB203580