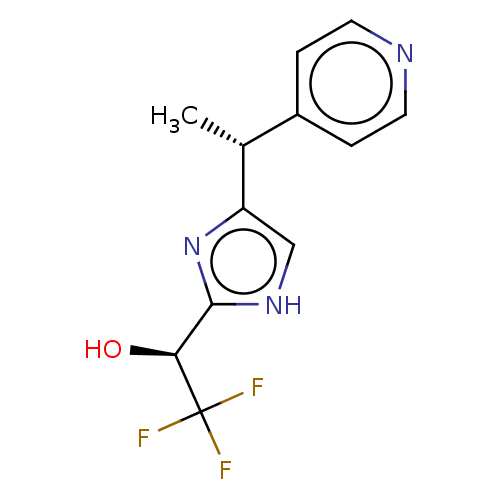
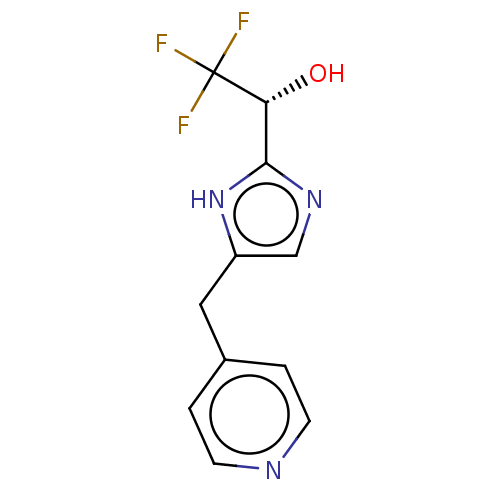
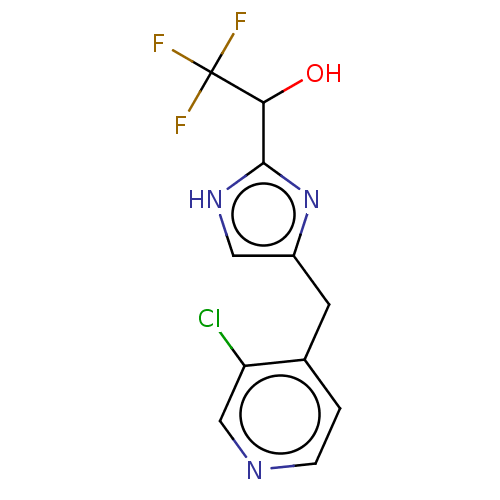
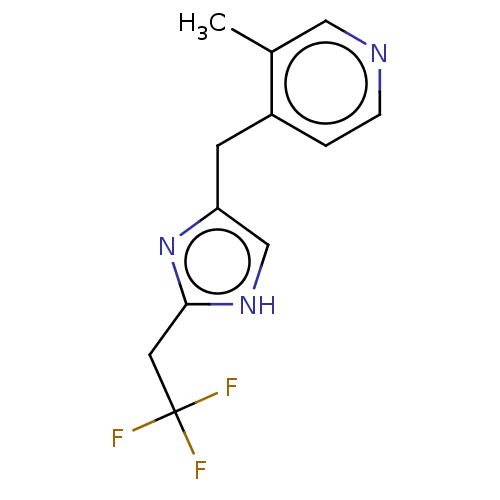
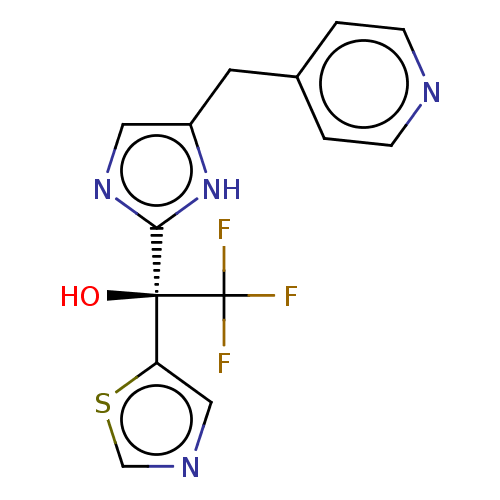
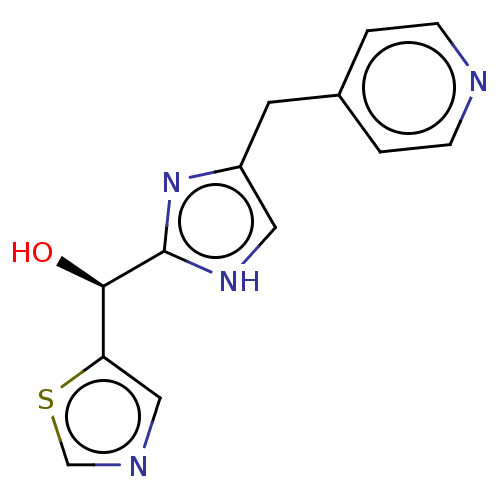
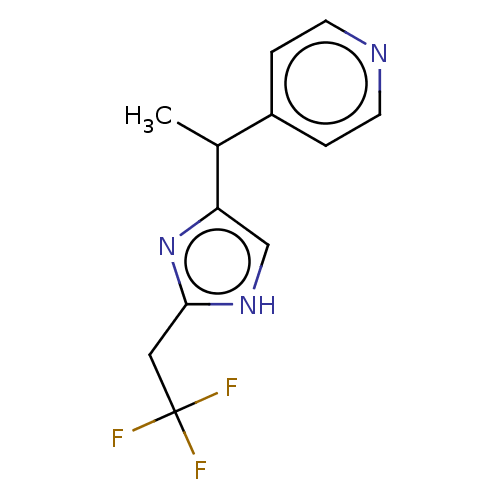
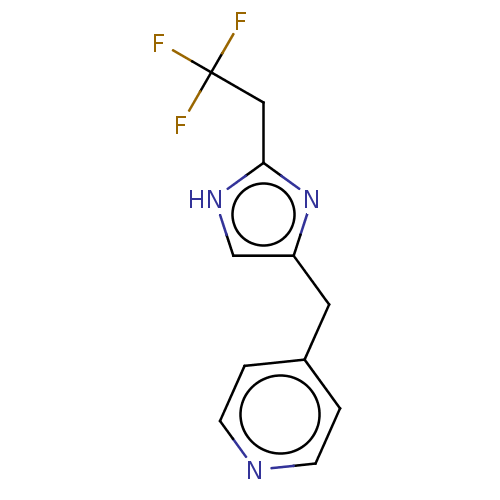
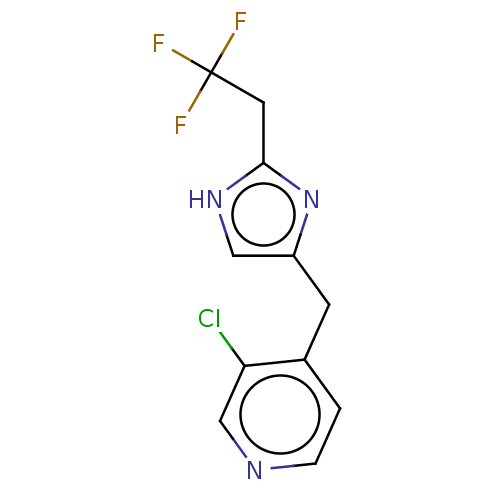
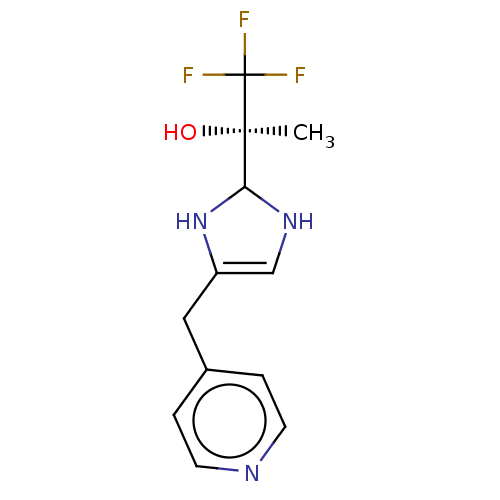
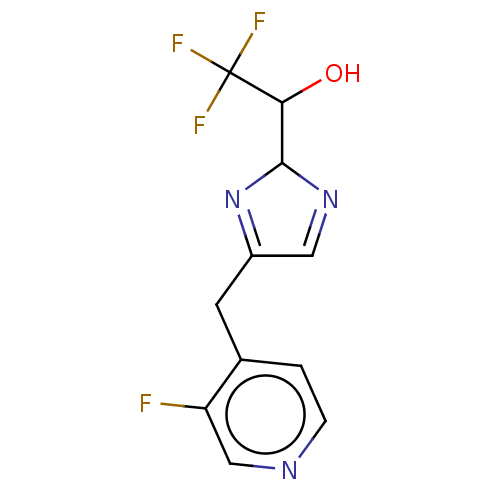
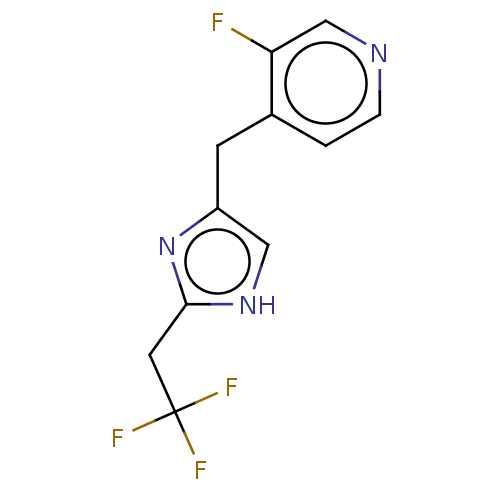
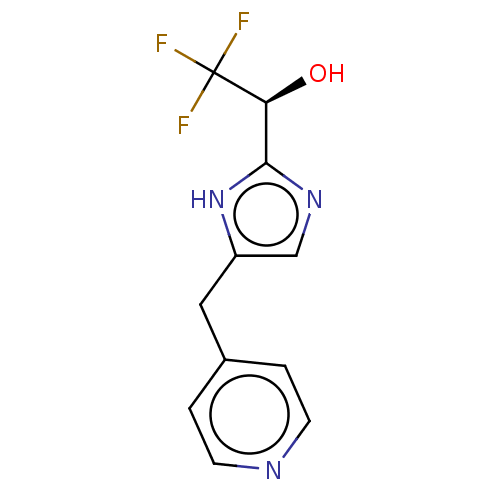
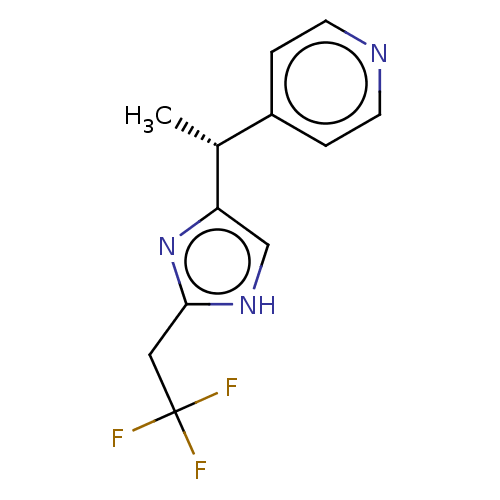
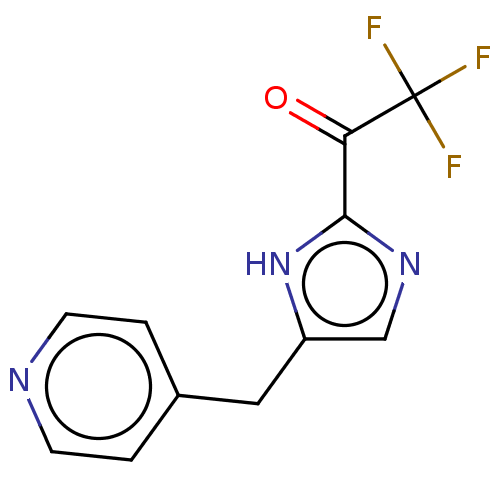
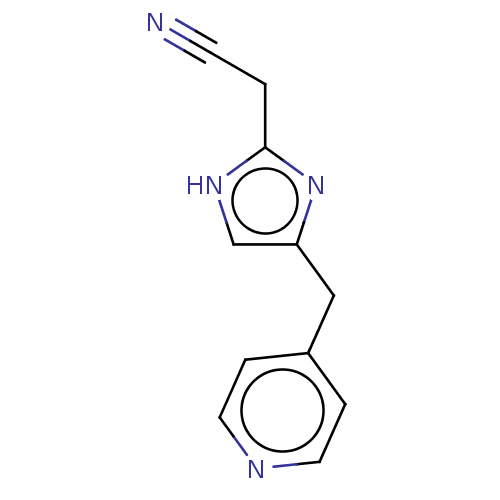
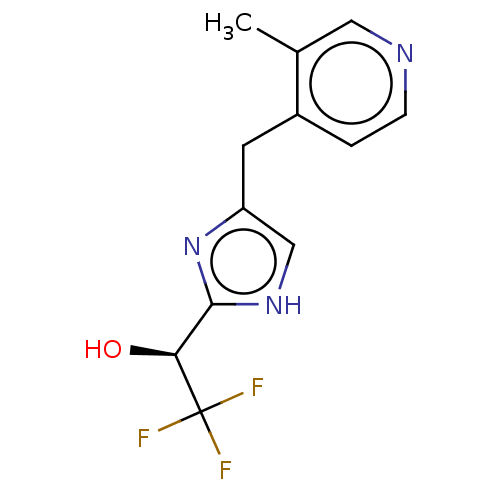
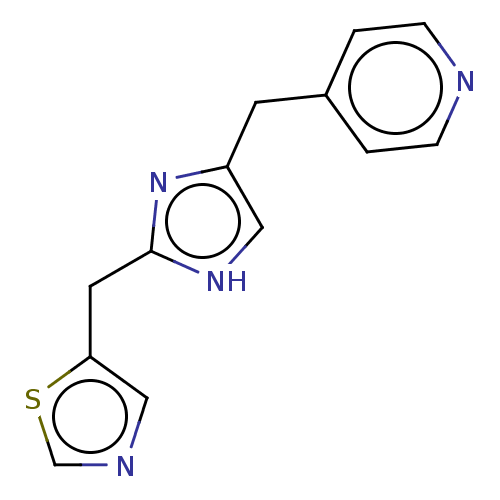
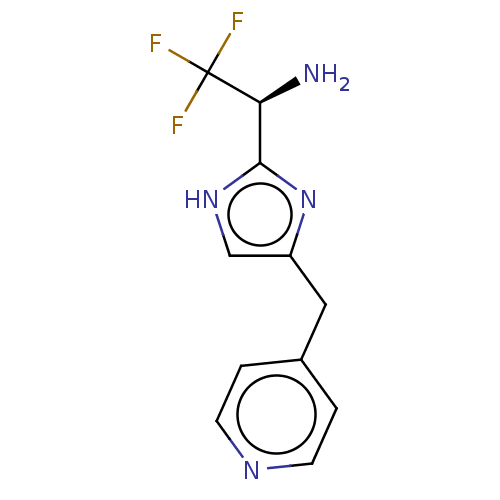
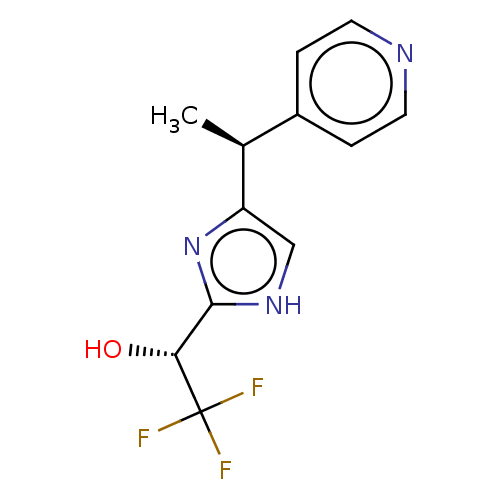
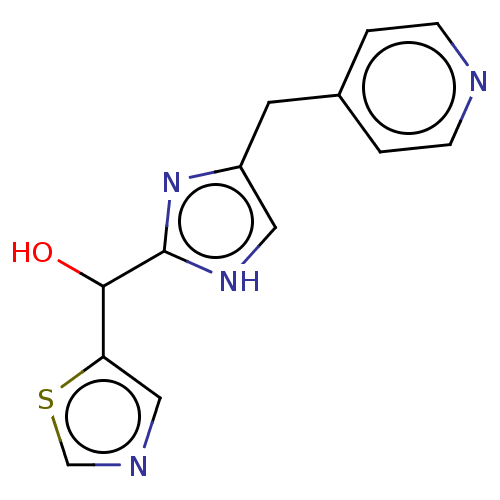
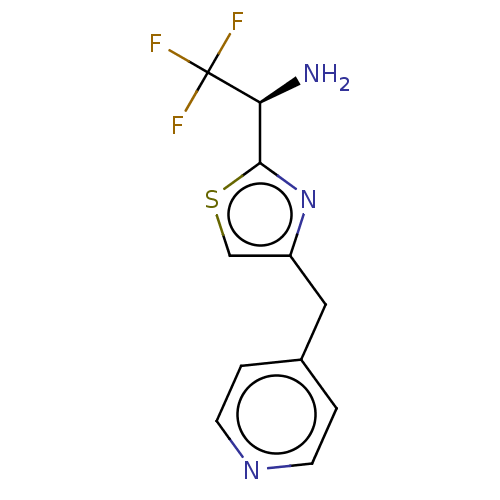
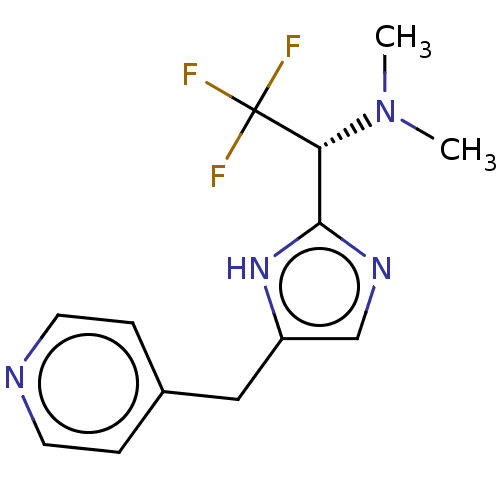
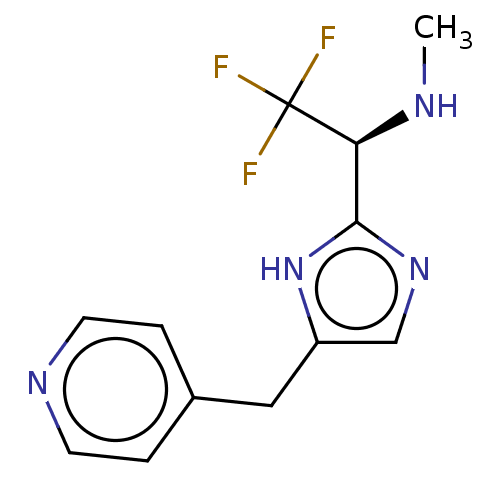
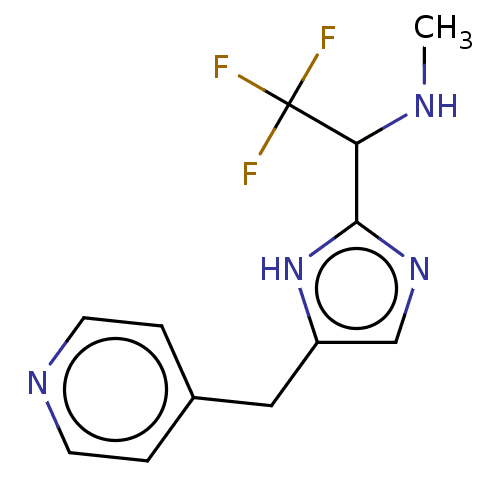
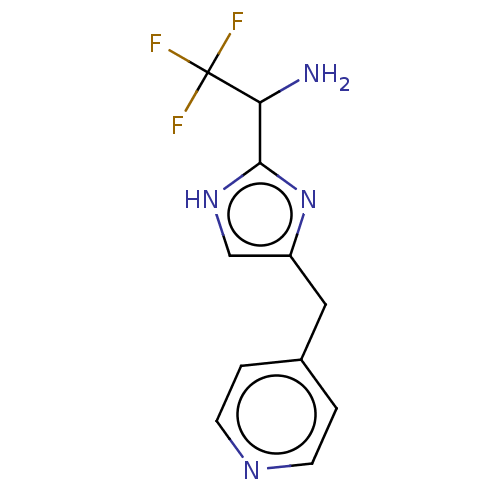
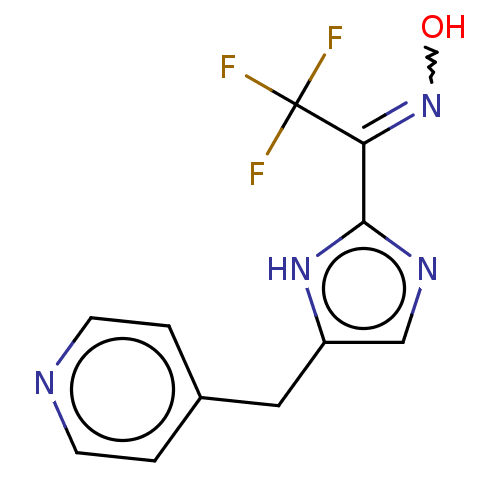
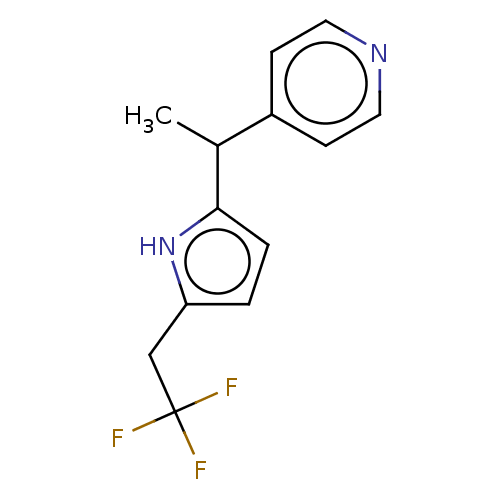
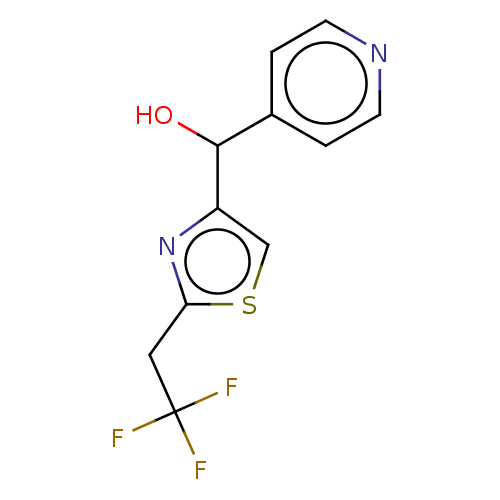
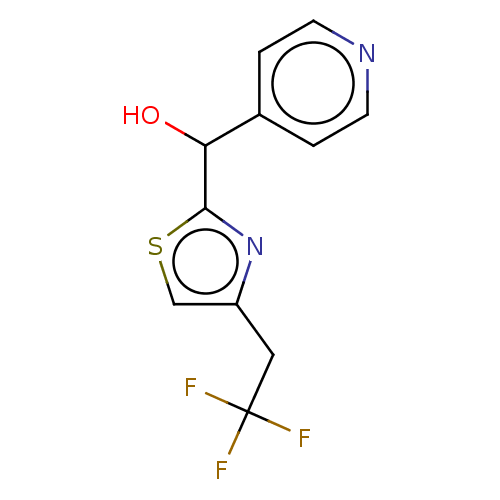
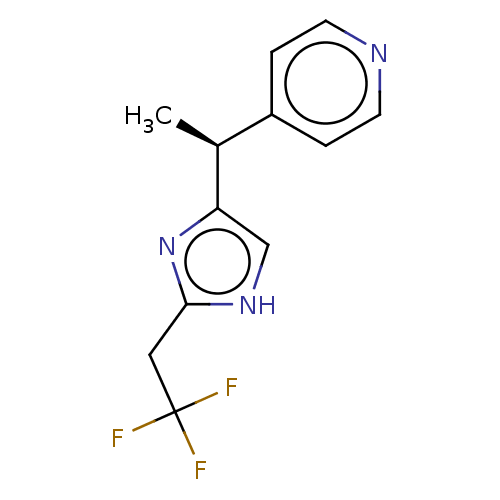
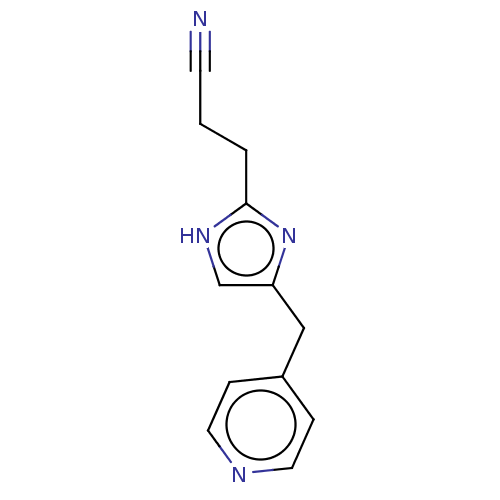
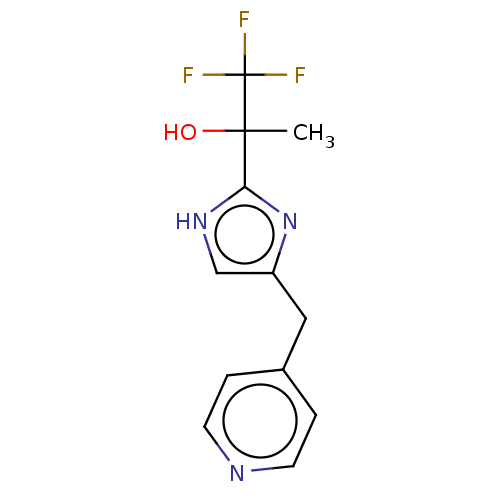
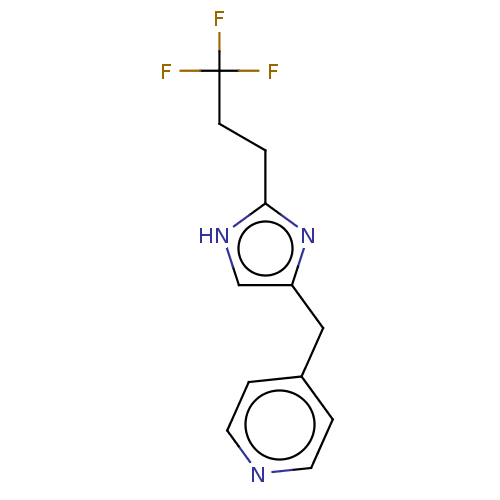
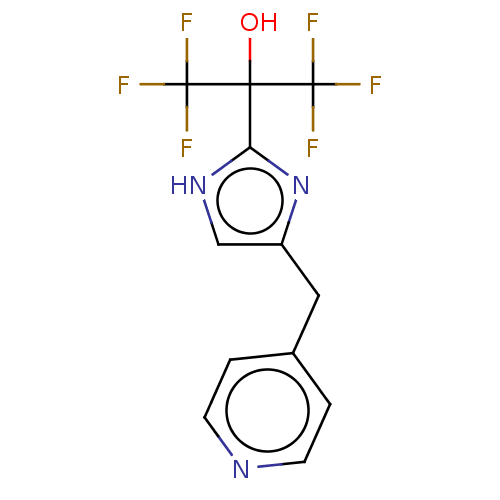
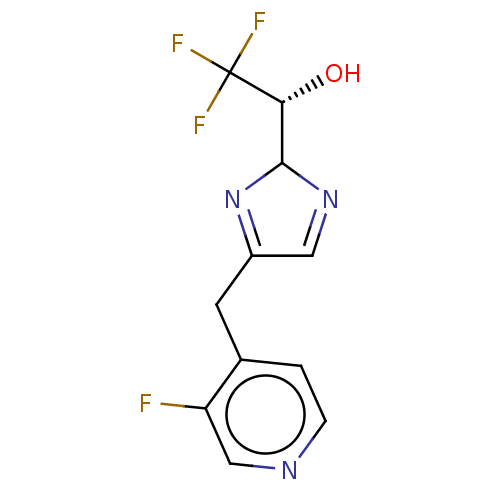
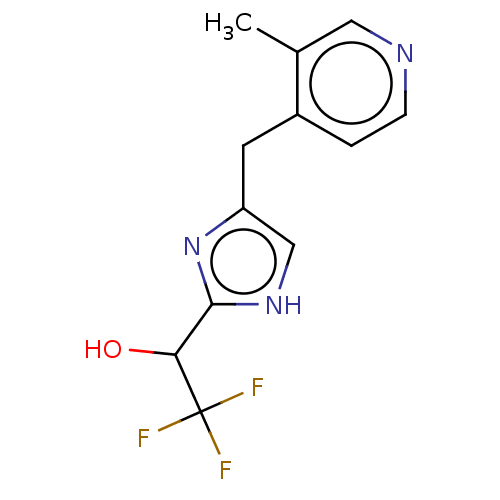
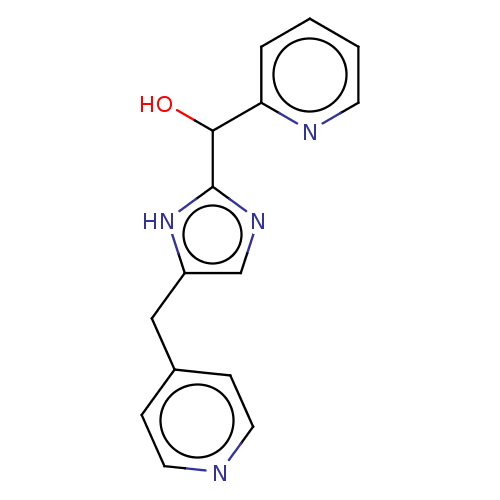
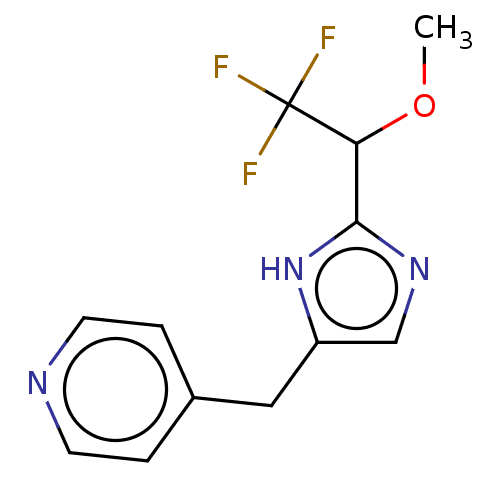
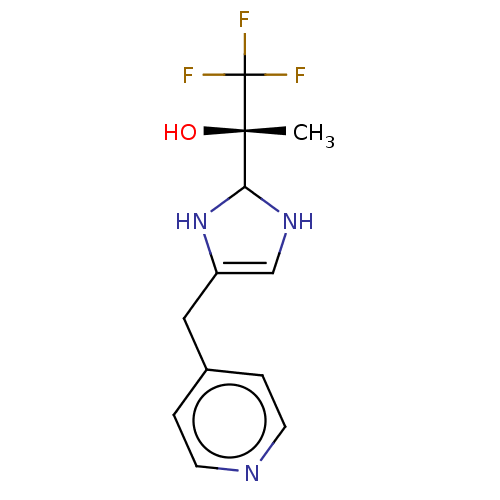
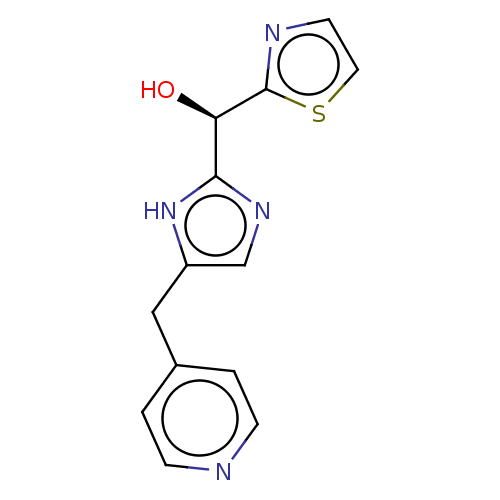
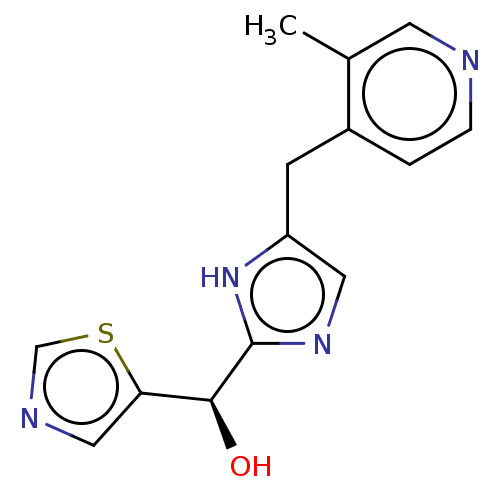
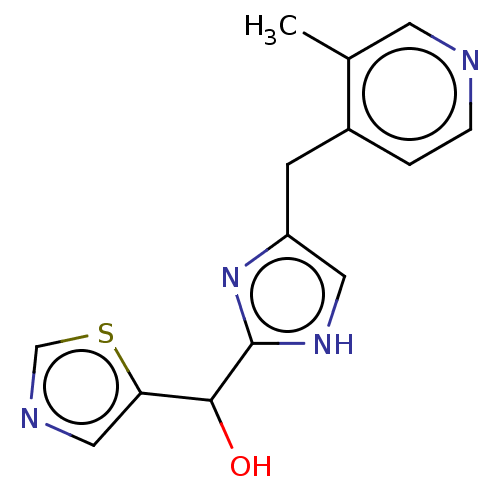
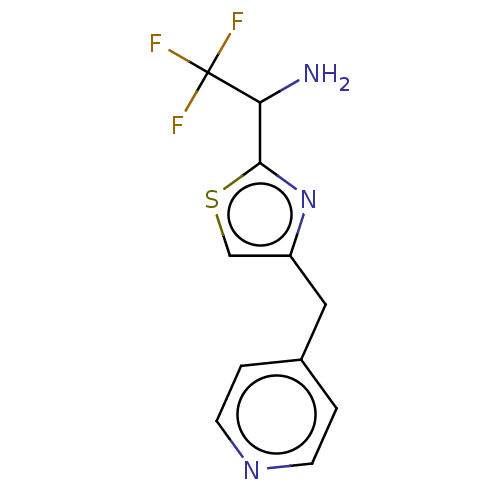
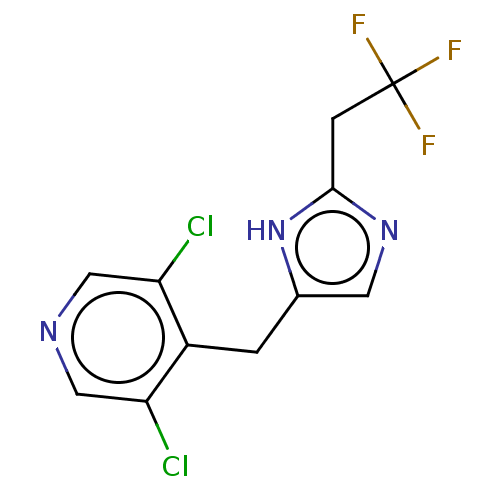
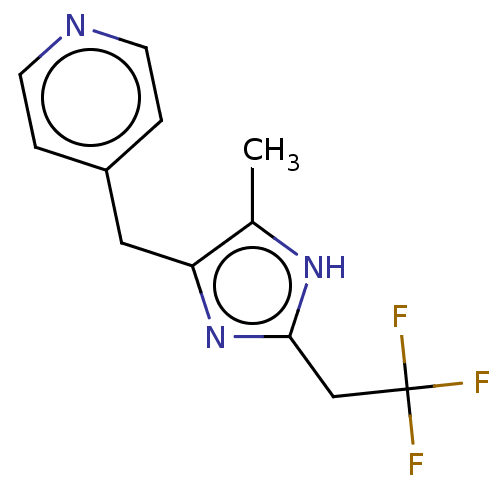
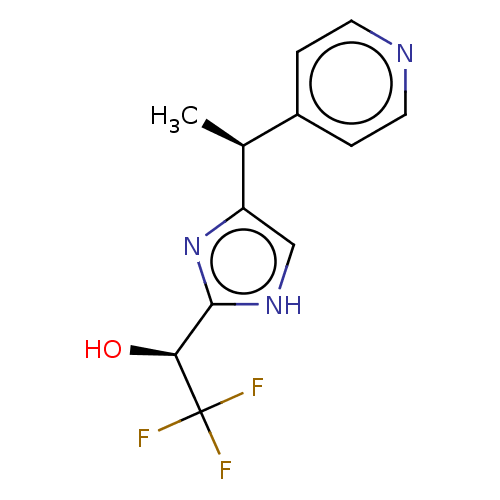
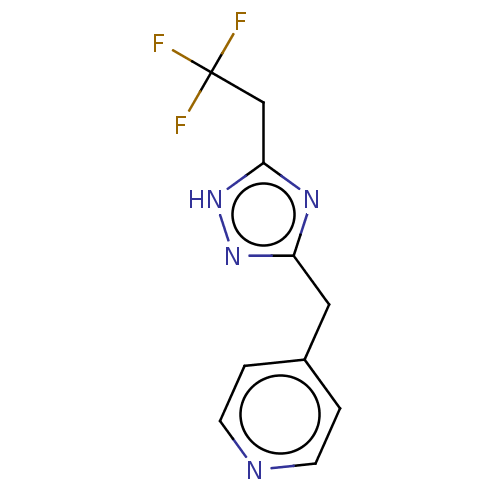
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: <1.00E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair