TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibitory activity against Angiotensin I converting enzyme (ACE) from human blood serumMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 0.100nMAssay Description:Inhibition of human serum ACEMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Angiotensin I converting enzyme (ACE) in Bothrops jararaca venomMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibitory activity against angiotensin converting enzyme (ACE)More data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 1.20nMAssay Description:Inhibition of Angiotensin I converting enzymeMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 3.98nMAssay Description:Inhibition of angiotensin I converting enzyme in silicoMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 4.70nMAssay Description:Inhibitory activity against angiotensin I converting enzyme (ACE)Checked by AuthorMore data for this Ligand-Target Pair
TargetAngiotensin-converting enzyme(Homo sapiens (Human))
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Institute Of Biomedical Chemistry Of Russian Academy Of Medical Sciences
Curated by ChEMBL
Affinity DataIC50: 7.90nMAssay Description:Inhibition of ACE (unknown origin) using Hippuryl-L-Histidyl-L-Leucine as substrate after 60 mins by colorimetric methodMore data for this Ligand-Target Pair

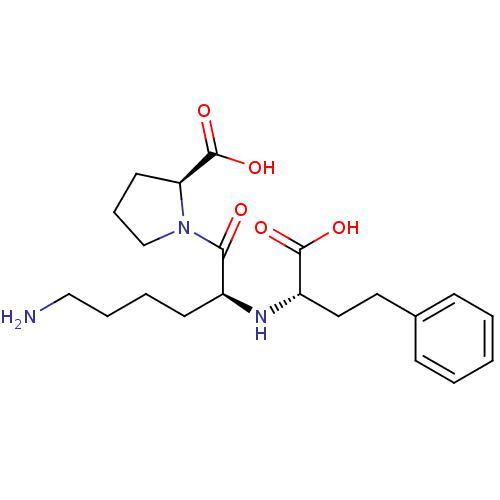
 3D Structure (crystal)
3D Structure (crystal)