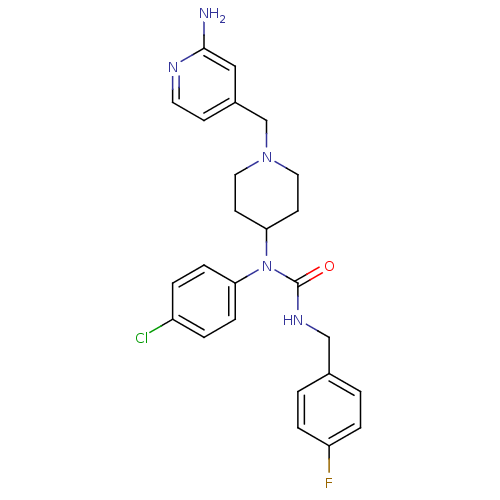
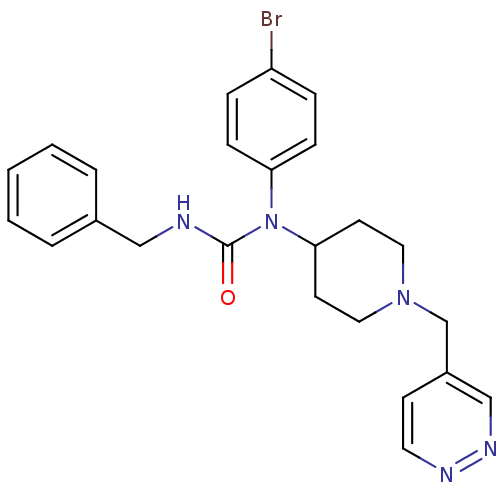
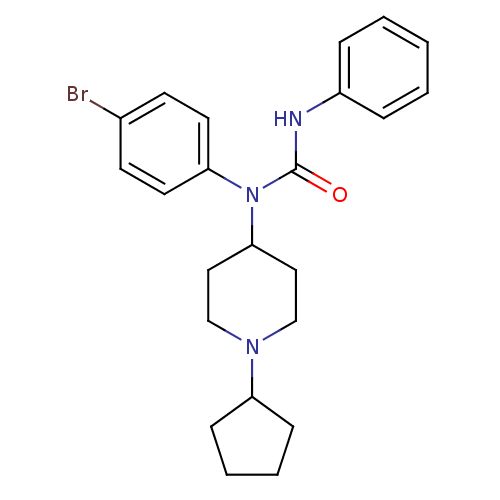
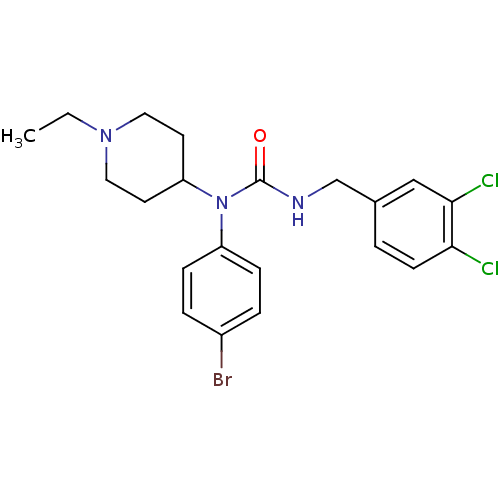
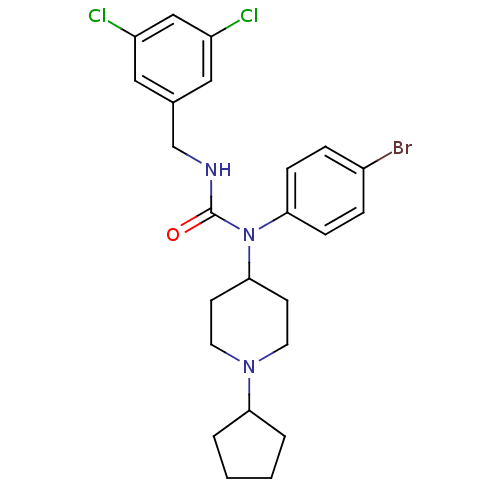
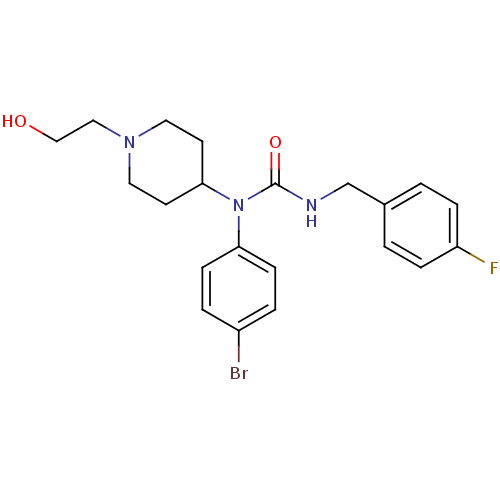
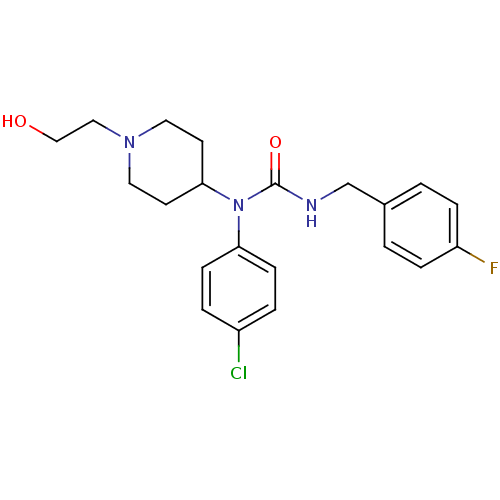
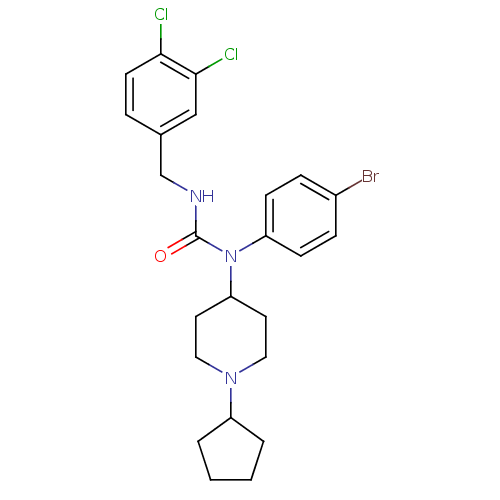
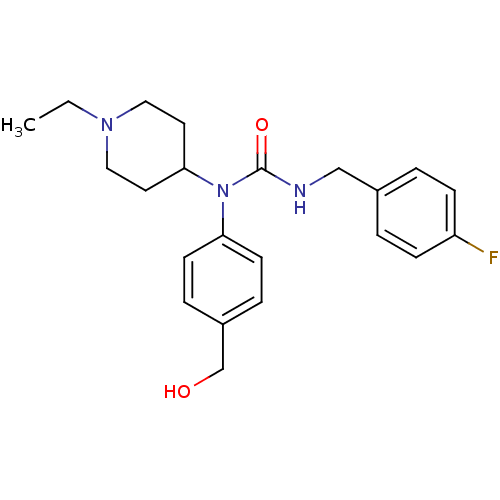
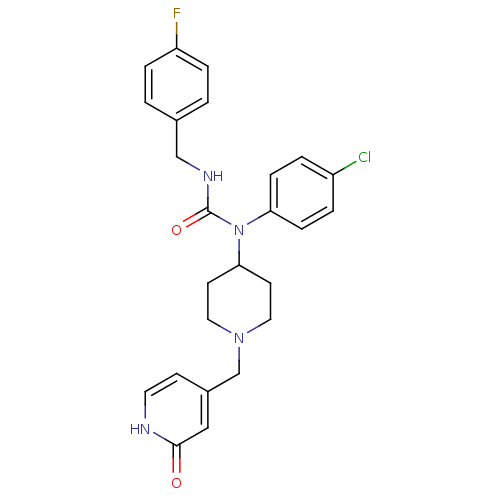
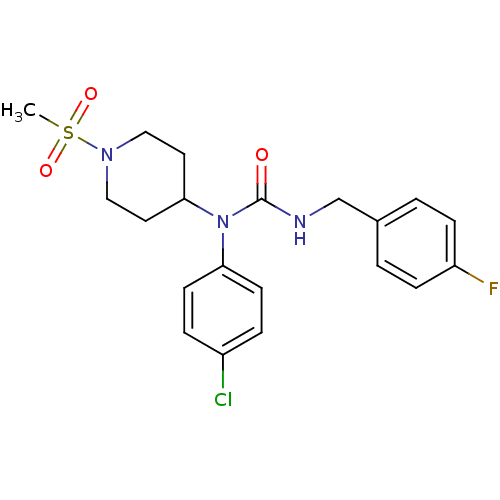
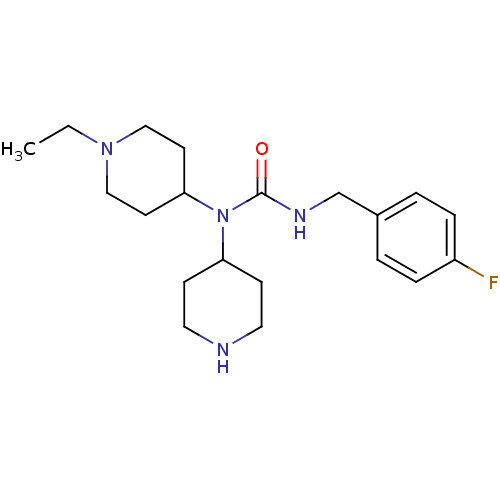
Report error Found 41 Enz. Inhib. hit(s) with all data for entry = 50031500
Affinity DataKi: 2nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 7nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 10nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 14nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 16nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 23nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 36nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 41nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 47nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 49nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 61nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
Affinity DataKi: 67nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 178nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 188nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
Affinity DataKi: 191nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 202nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 384nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
Affinity DataKi: 447nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 560nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 624nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: 858nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
Affinity DataKi: >1.00E+3nMAssay Description:Antagonist activity at human recombinant histamine 3 receptorMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 2.31E+3nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 3.81E+3nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 6.65E+3nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 7.88E+3nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 9.19E+3nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.20E+4nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.27E+4nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Merck Research Laboratories
Curated by ChEMBL
Merck Research Laboratories
Curated by ChEMBL
Affinity DataIC50: 1.38E+4nMAssay Description:Inhibition of human ERG by ion works assayMore data for this Ligand-Target Pair