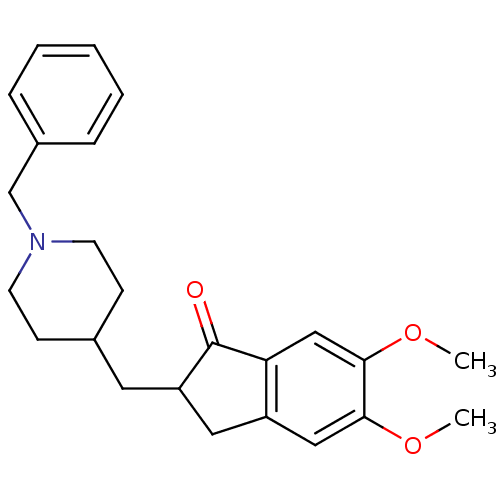
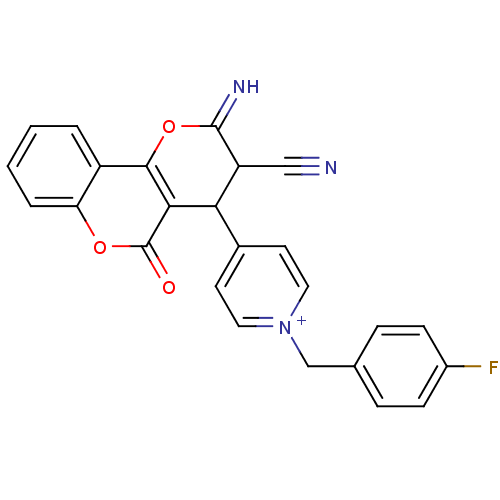
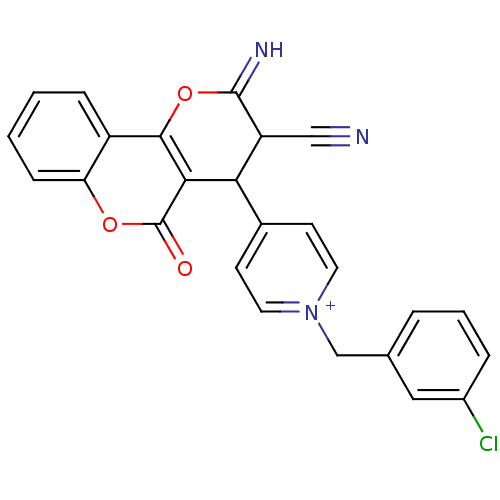
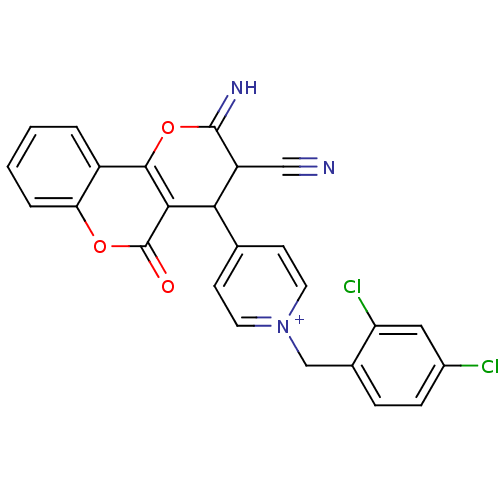
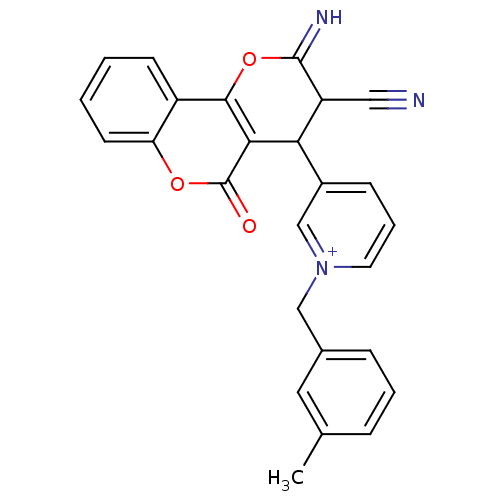
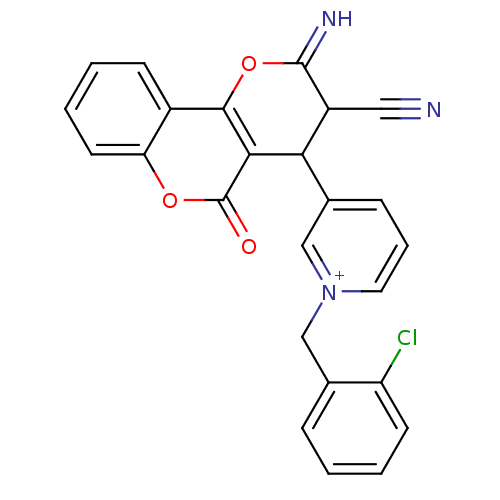
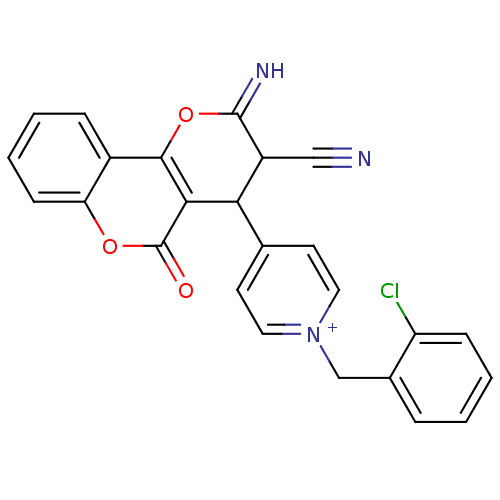
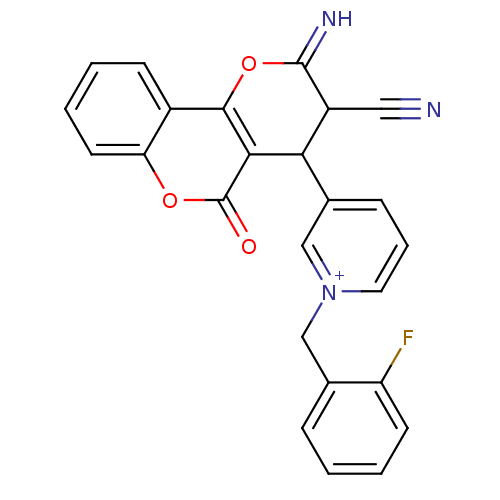
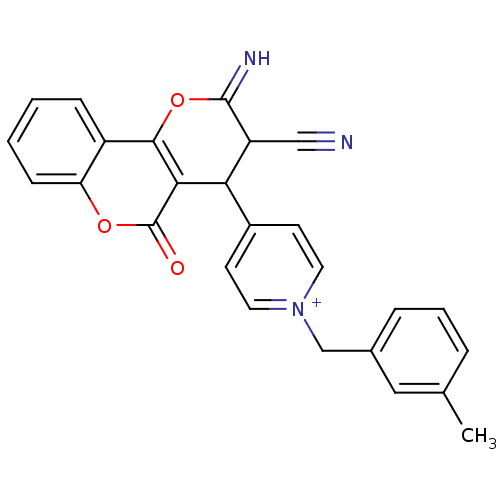
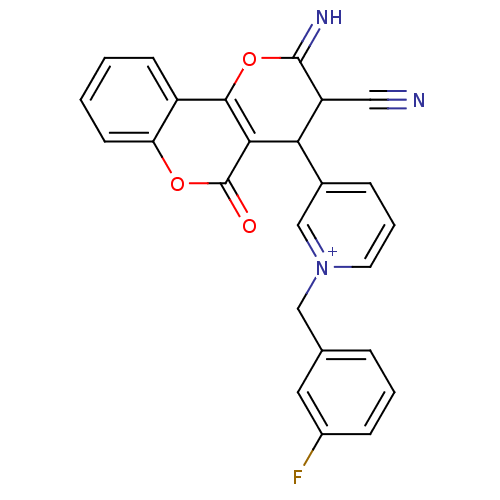
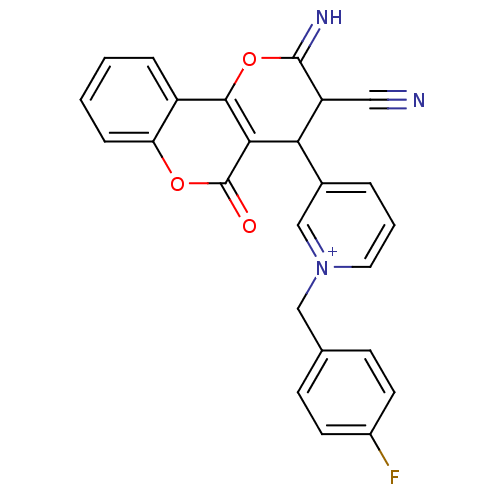
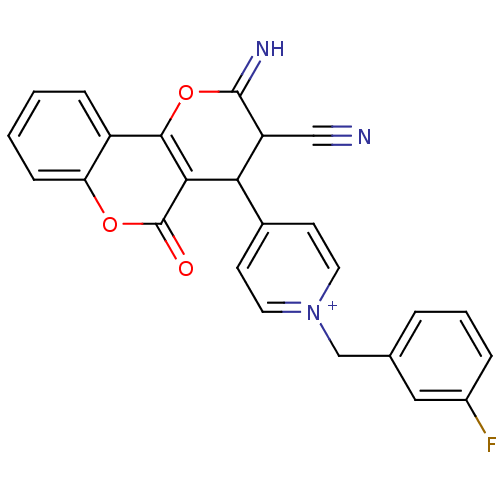
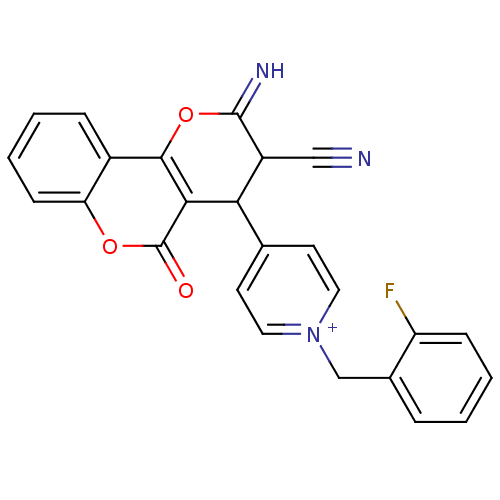
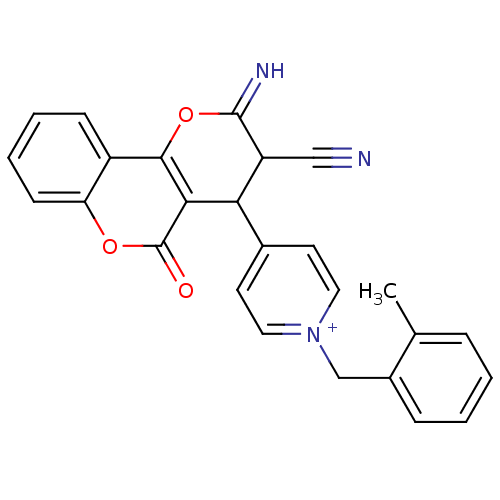
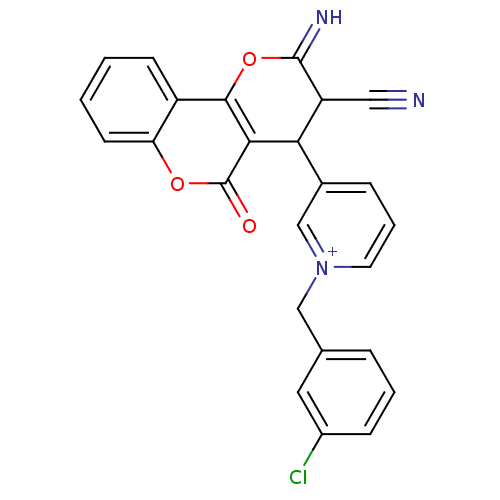
Report error Found 32 Enz. Inhib. hit(s) with all data for entry = 50043395
Affinity DataIC50: 14nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 480nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 566nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 790nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 831nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.27E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.50E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.80E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.84E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.17E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.80E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.90E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.70E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.38E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+3nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 7.40E+3nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.12E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.43E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.78E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.18E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.60E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 3.84E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+4nMAssay Description:Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate after 2 mins by Ellman's methodMore data for this Ligand-Target Pair