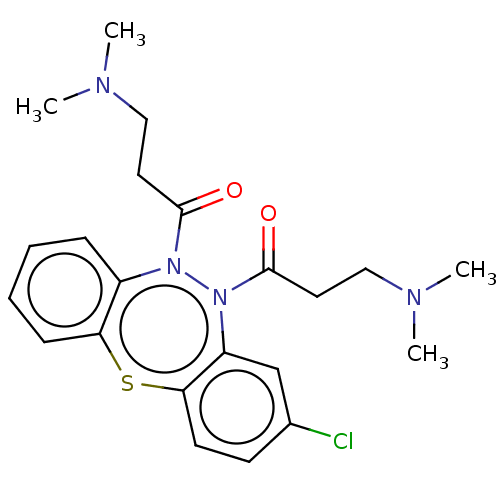
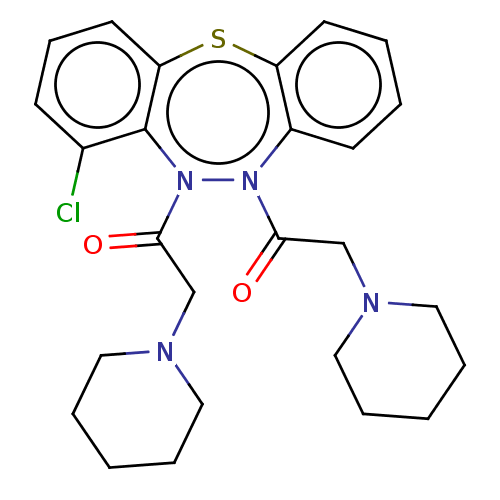
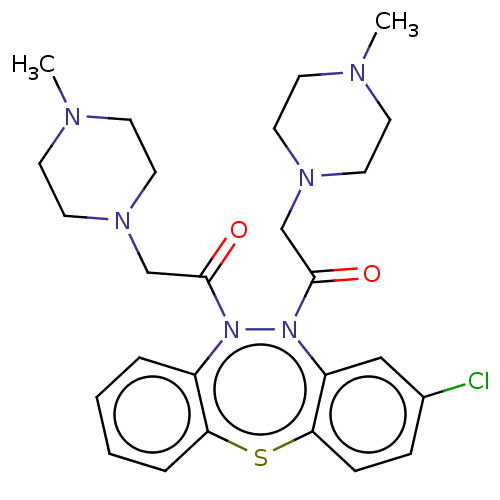
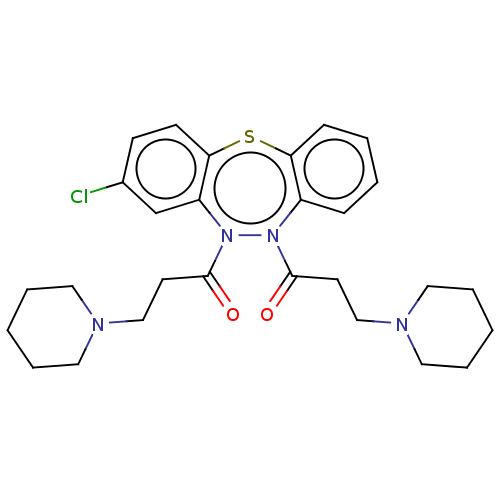
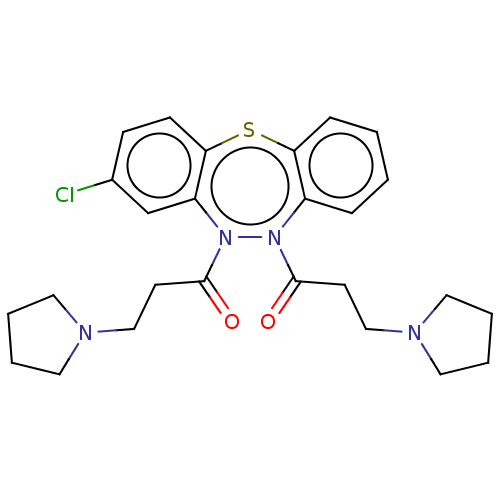
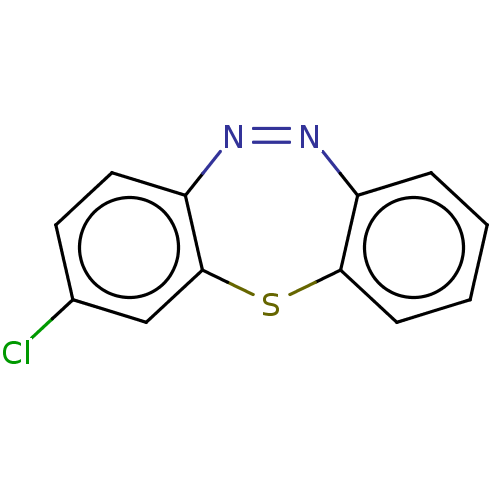
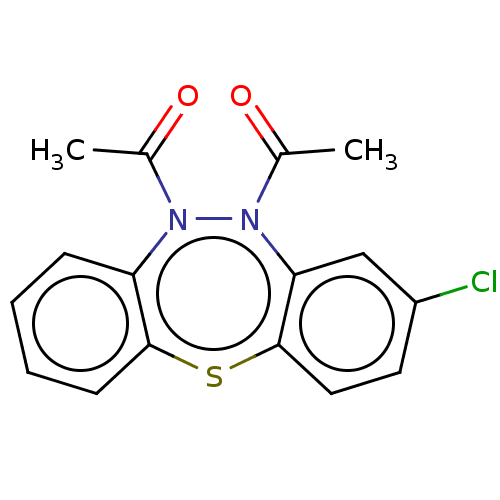
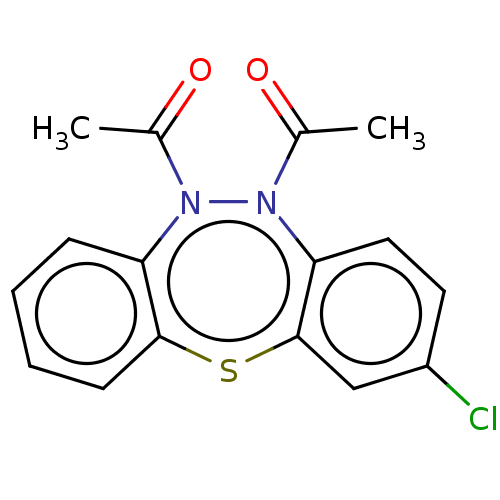
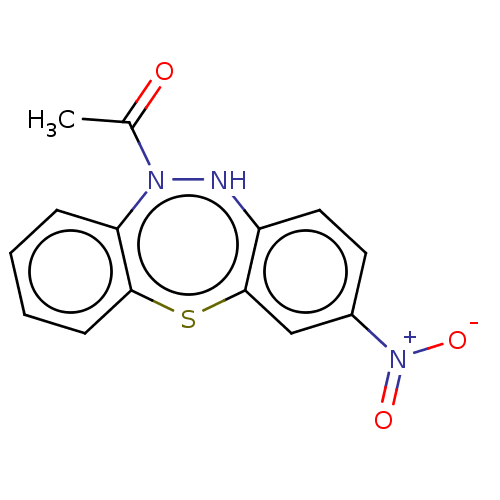
Affinity DataIC50: 10nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: >1.00E+5nMAssay Description:Inhibition of horse serum BuChE using butyrylthiocholine as substrate by UV spectroscopic analysisMore data for this Ligand-Target Pair

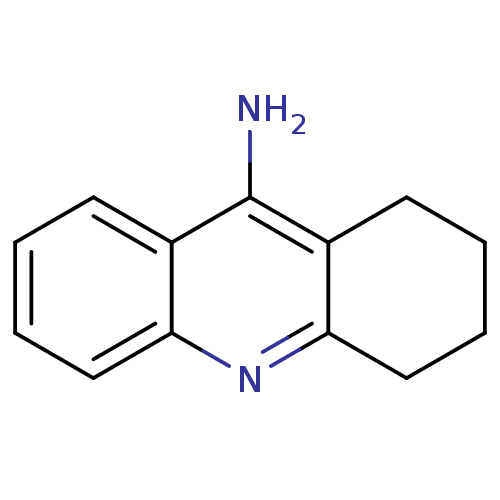
 3D Structure (crystal)
3D Structure (crystal)