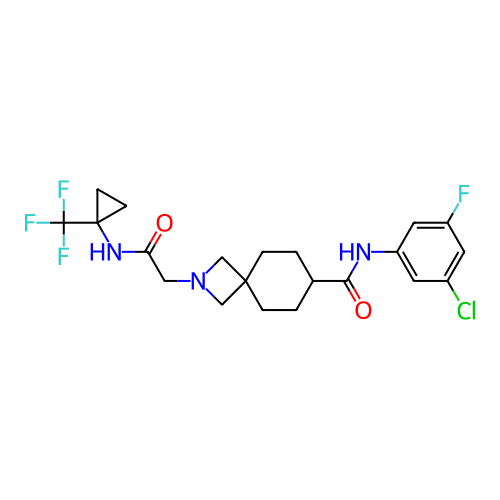
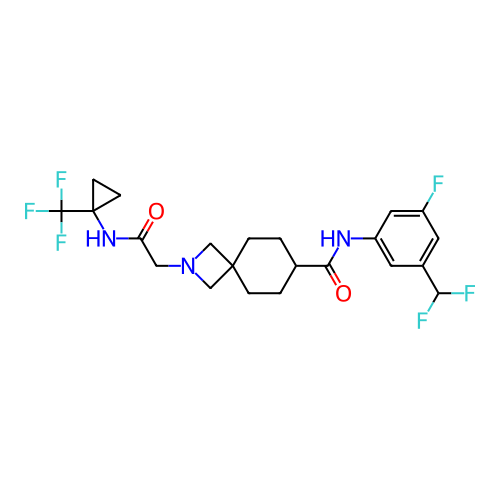
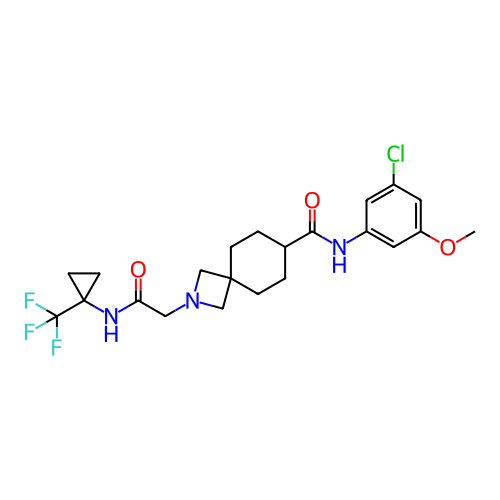
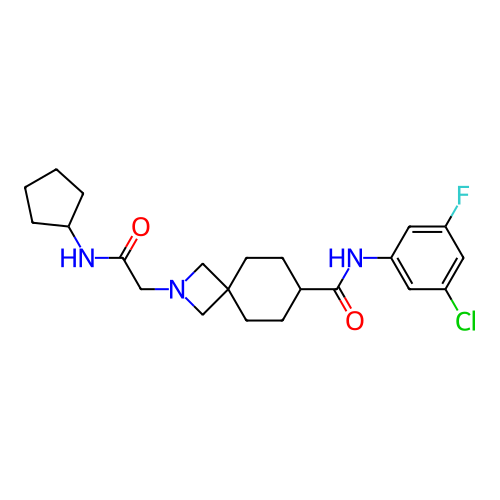
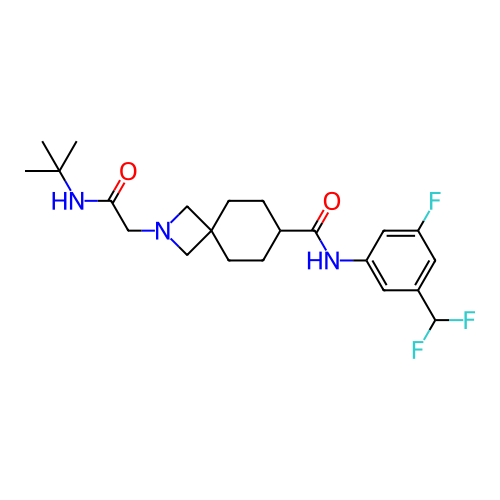
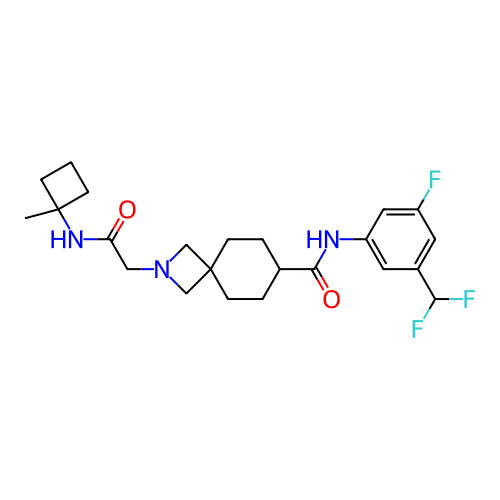
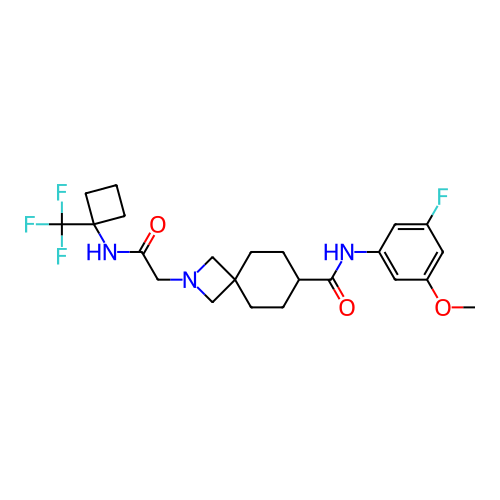
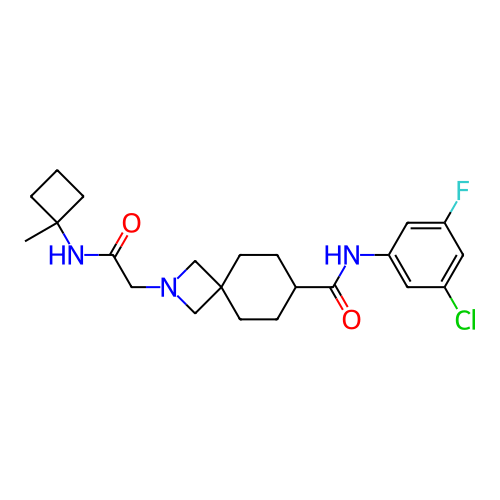
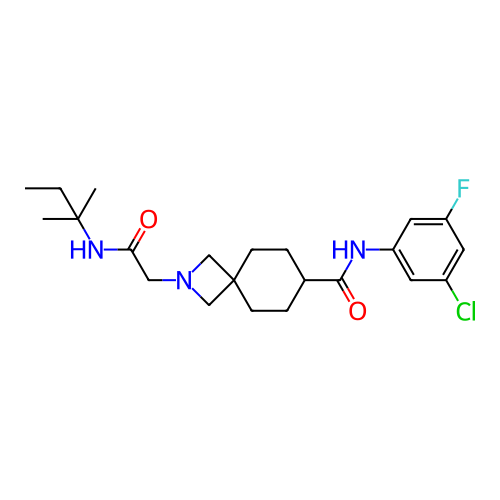
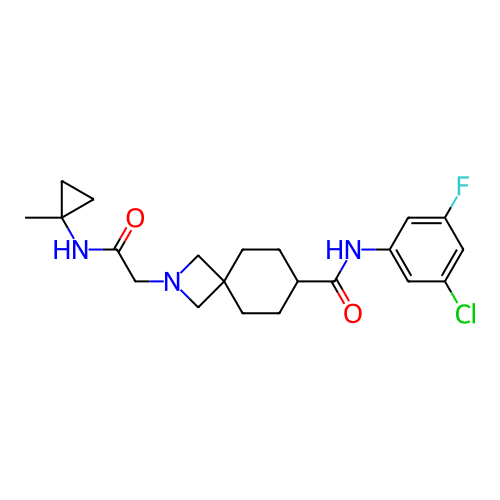
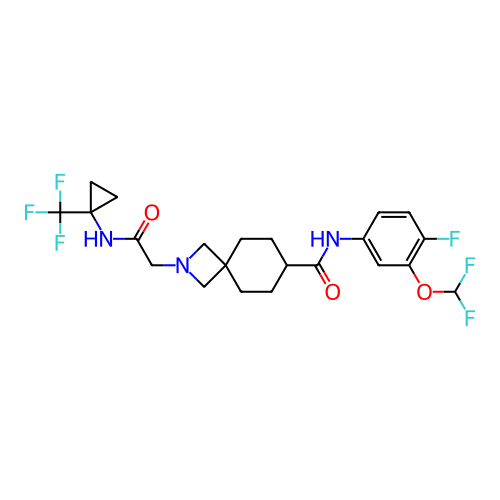
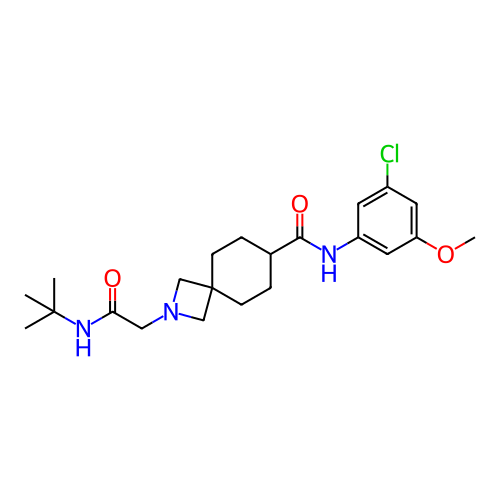
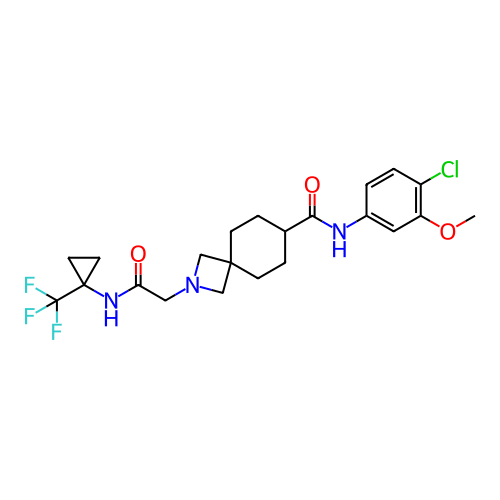
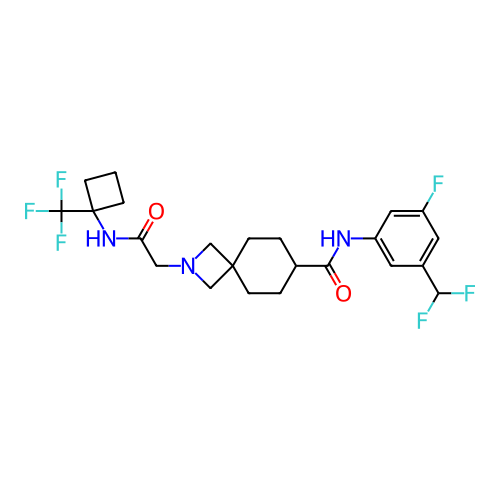
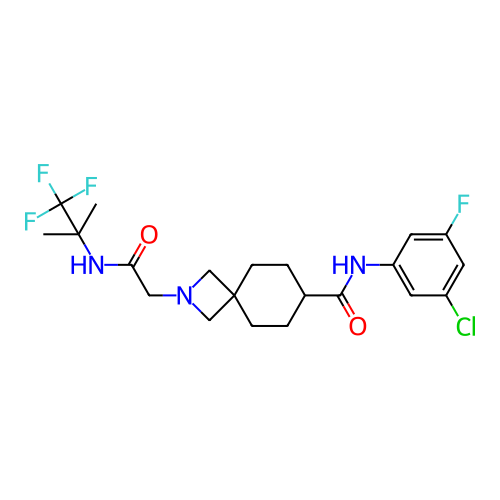
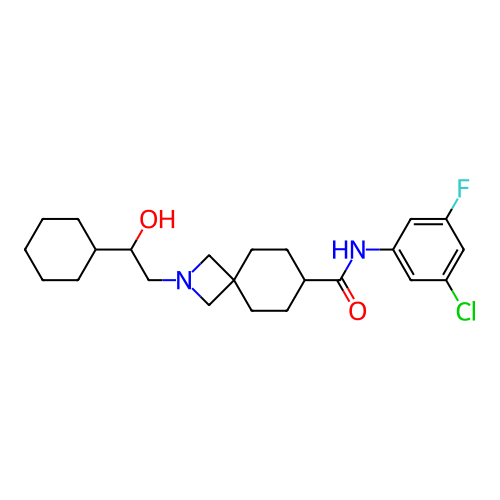
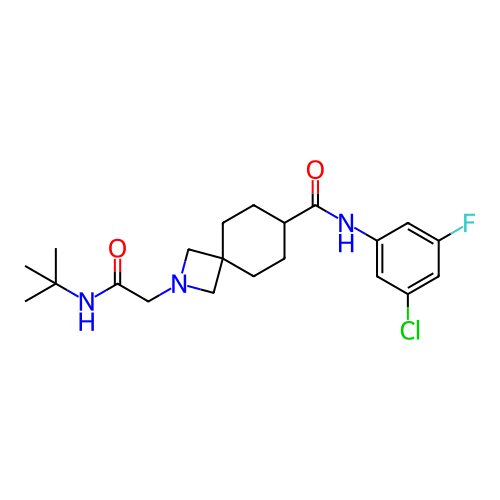
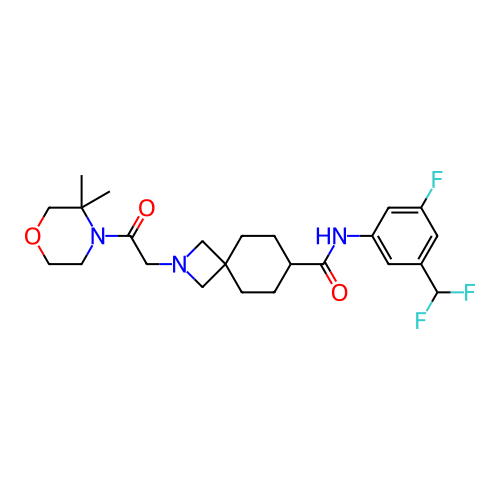
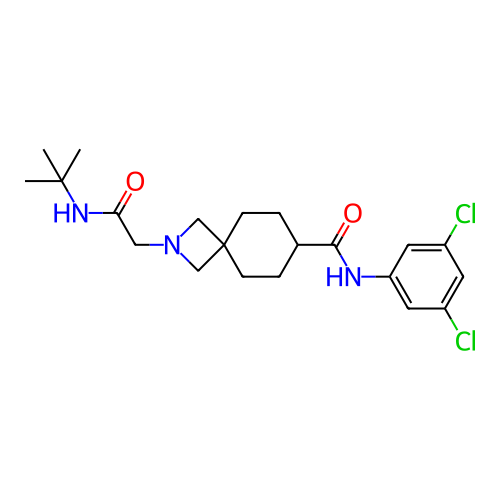
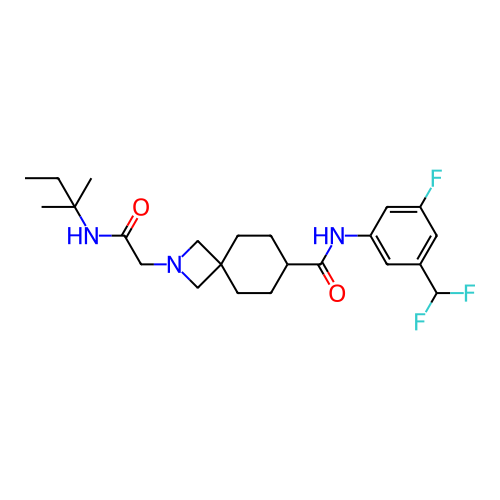
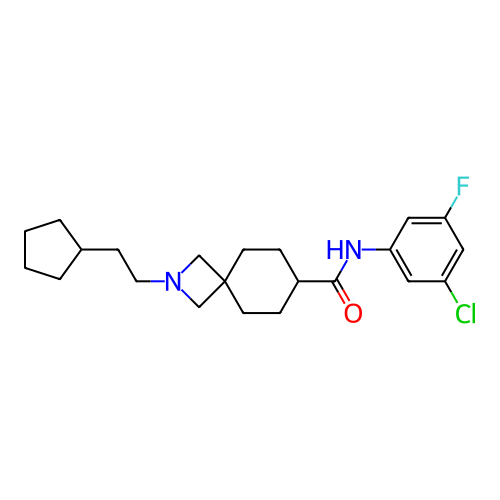
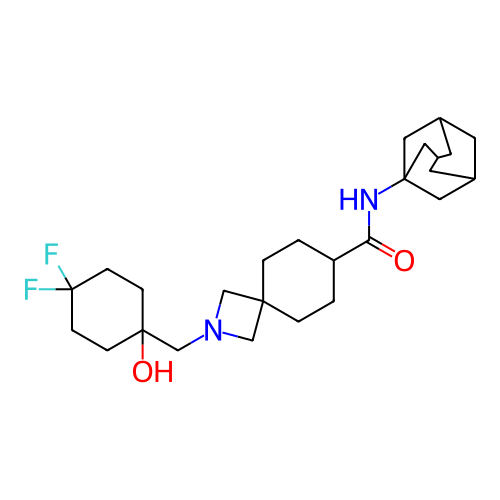
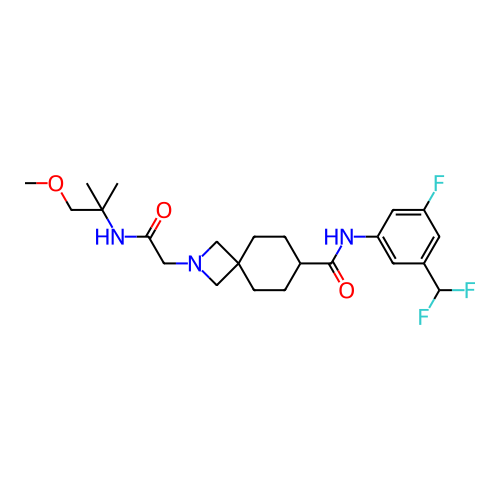
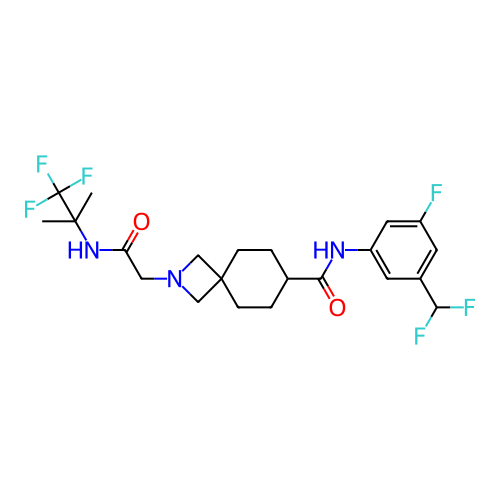
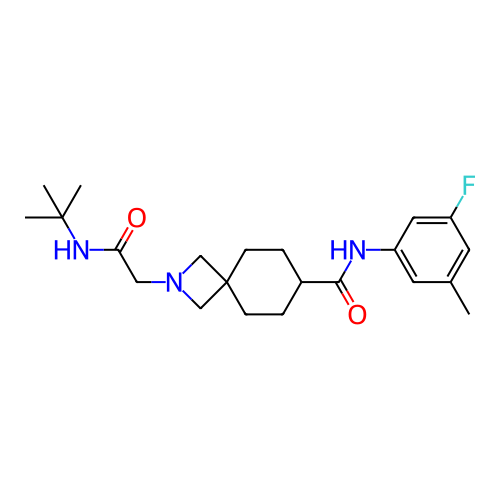
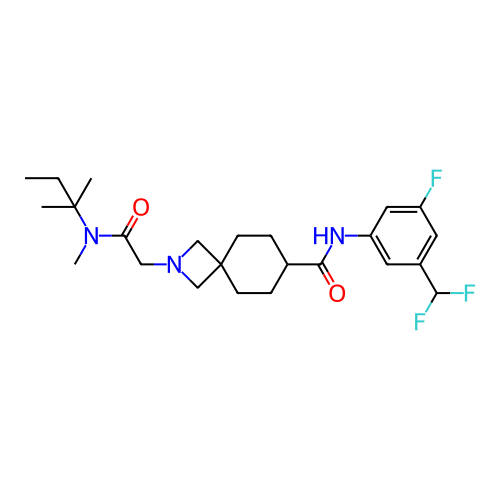
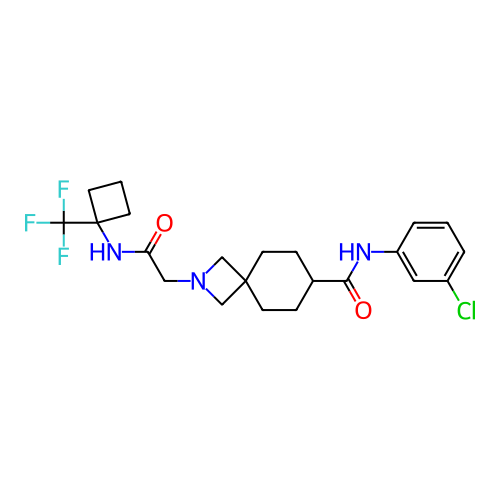
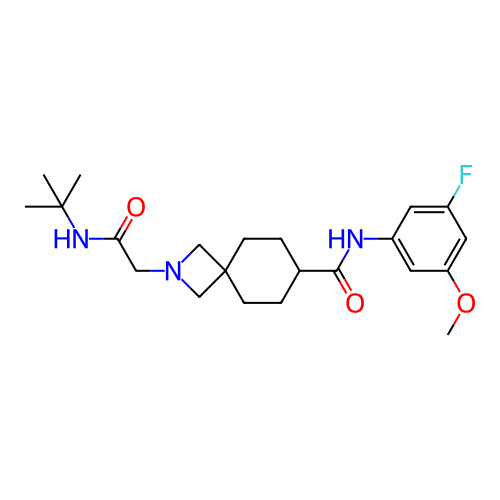
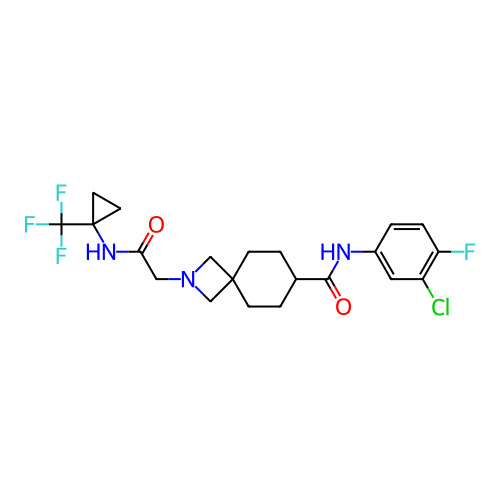
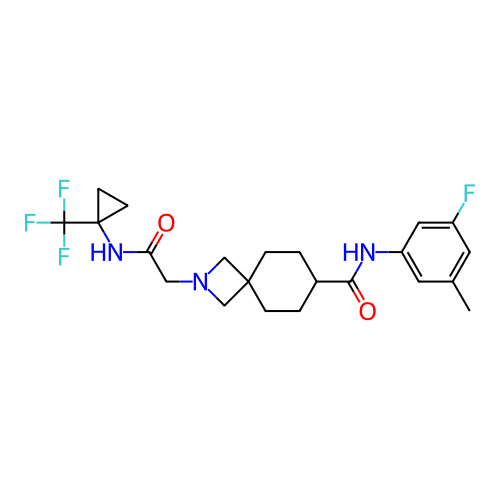
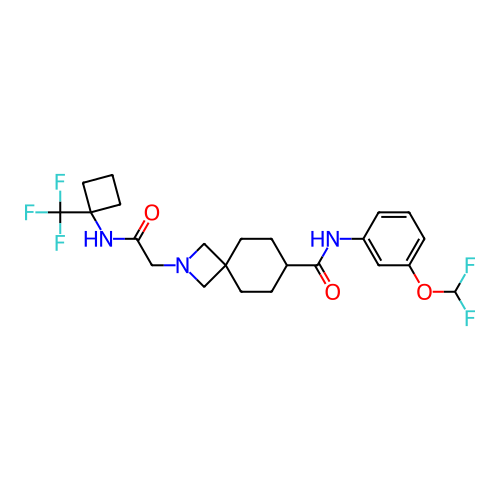
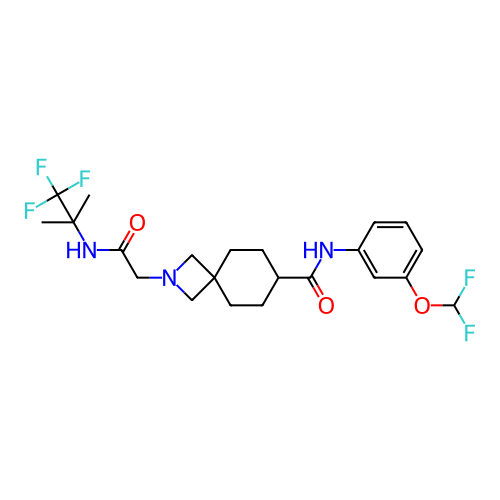
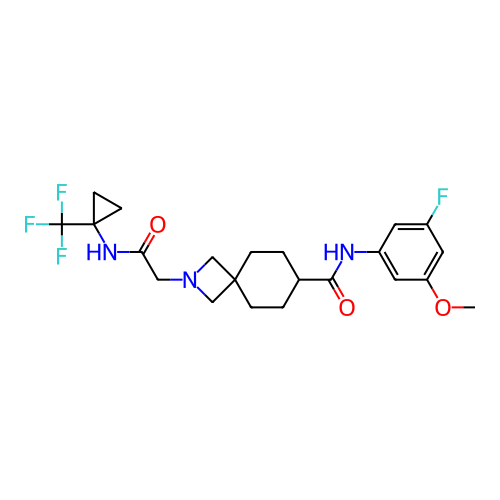
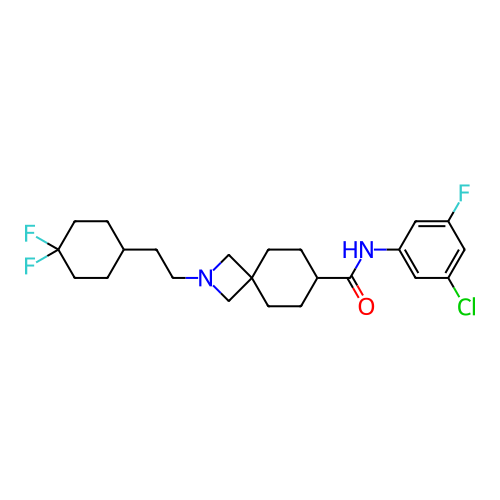
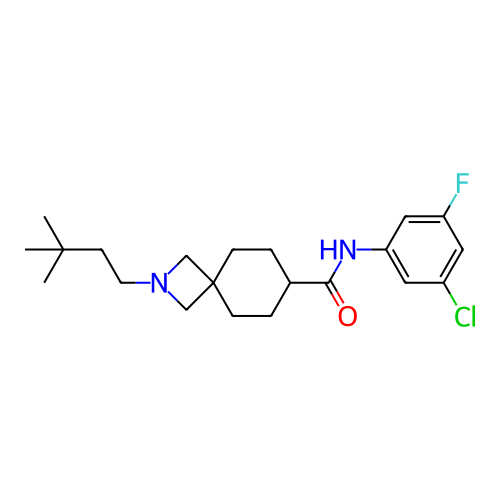
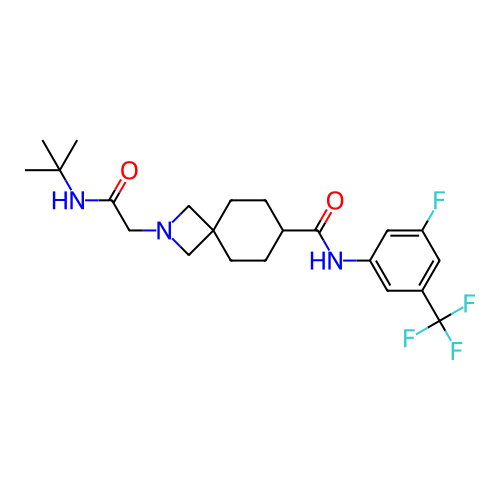
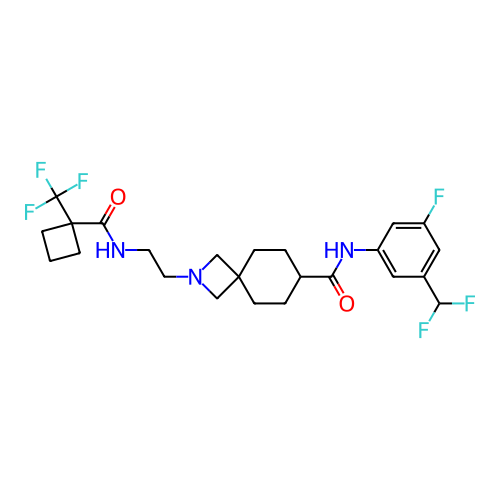
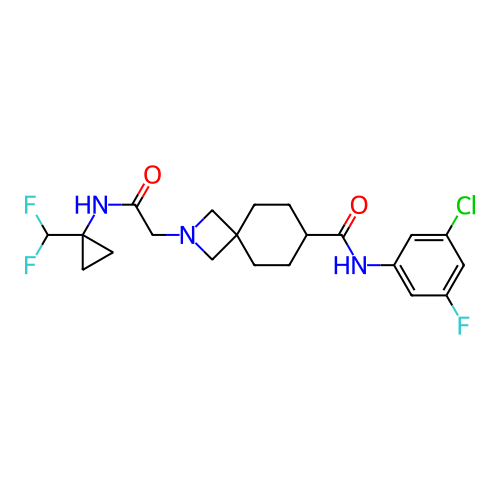
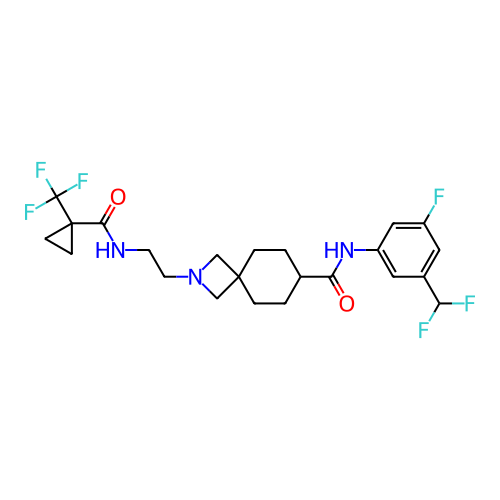
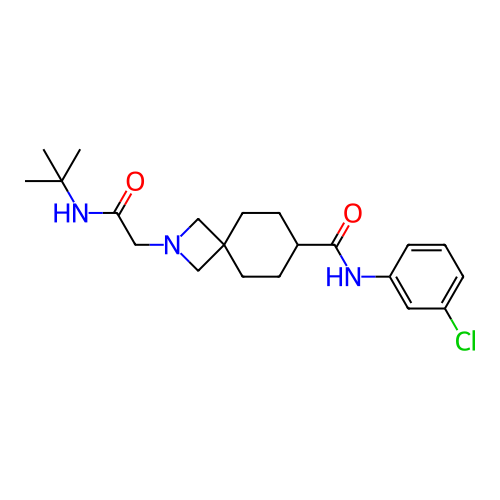
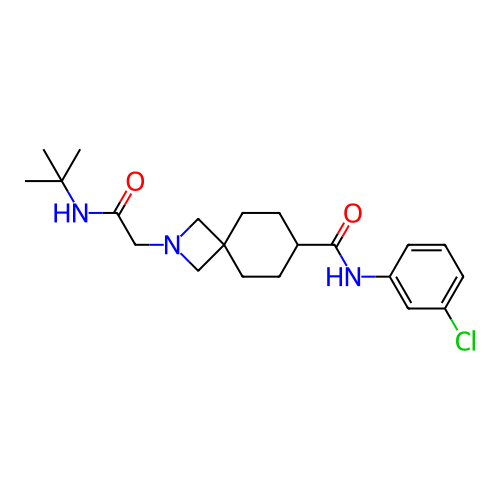
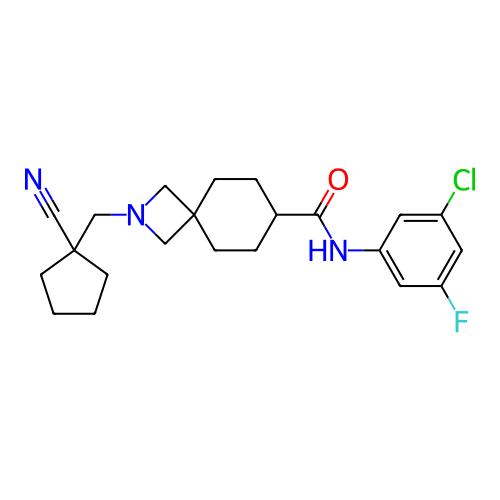
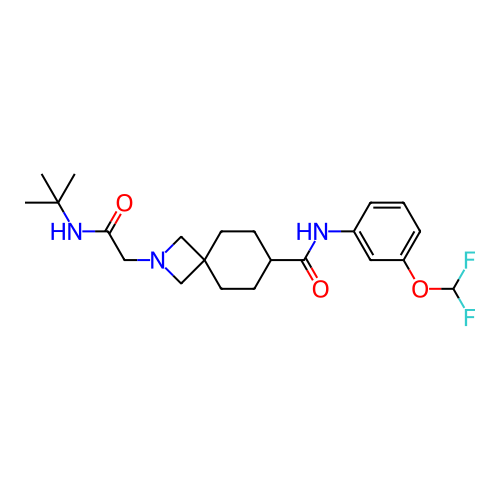
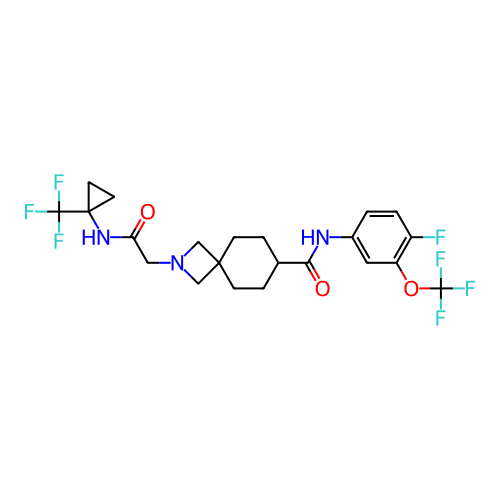
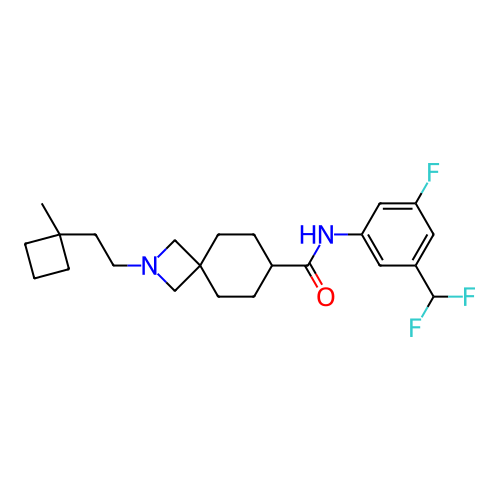
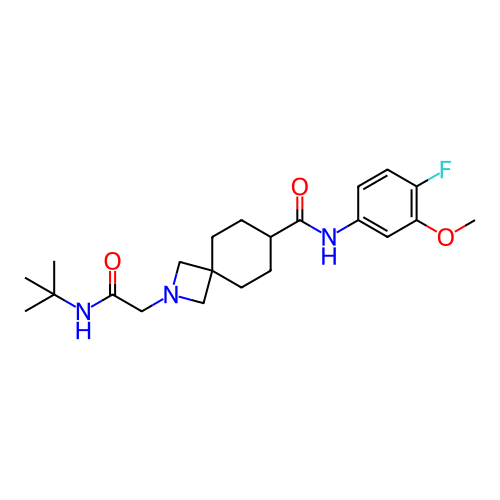
Report error Found 190 Enz. Inhib. hit(s) with all data for entry = 13056
Affinity DataIC50: 59.7nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 78.2nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 78.2nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 78.2nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 78.5nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 102nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 110nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 112nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 118nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 129nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 130nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 139nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 142nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 146nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 149nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 159nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 162nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 163nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 170nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 174nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 176nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 182nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 182nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 188nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 191nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 192nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 196nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 199nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 204nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 218nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 219nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 221nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 231nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 235nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 238nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 239nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 252nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 254nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 261nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 283nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 284nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 294nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 294nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 296nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 296nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 297nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:The compound plate was created at 2× concentrated in the extracellular solution. The compound was diluted to 1:2 when added to the recording well. Th...More data for this Ligand-Target Pair