Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Potassium voltage-gated channel subfamily H member 2
Ligand
BDBM50462035
Substrate
n/a
Meas. Tech.
ChEMBL_1772472 (CHEMBL4224584)
IC50
410±n/a nM
Citation
 Li, L; Okumu, A; Dellos-Nolan, S; Li, Z; Karmahapatra, S; English, A; Yalowich, JC; Wozniak, DJ; Mitton-Fry, MJ Synthesis and anti-staphylococcal activity of novel bacterial topoisomerase inhibitors with a 5-amino-1,3-dioxane linker moiety. Bioorg Med Chem Lett 28:2477-2480 (2018) [PubMed] Article
Li, L; Okumu, A; Dellos-Nolan, S; Li, Z; Karmahapatra, S; English, A; Yalowich, JC; Wozniak, DJ; Mitton-Fry, MJ Synthesis and anti-staphylococcal activity of novel bacterial topoisomerase inhibitors with a 5-amino-1,3-dioxane linker moiety. Bioorg Med Chem Lett 28:2477-2480 (2018) [PubMed] Article More Info.:
Target
Name:
Potassium voltage-gated channel subfamily H member 2
Synonyms:
1,3-beta-glucan synthase component GLS2 | Cytochrome P450 3A4 | ERG | ERG1 | Eag-related protein 1 | Ether a-go-go related gene potassium channel (hERG) | Ether-a-go-go-related gene (HERG) | Ether-a-go-go-related gene potassium channel (hERG) | Ether-a-go-go-related gene potassium channel 1 | Ether-a-go-go-related gene potassium channel 1 (HERG) | Ether-a-go-go-related gene potassium channel 1 (hERG1) | Ether-a-go-go-related protein (hERG) | Ether-a-go-go-related protein 1 | Ether-a-go-go-related protein 1 (HERG) | H-ERG | HERG | KCNH2 | KCNH2_HUMAN | Potassium voltage-gated channel subfamily H member 2 (hERG) | Transcriptional regulator ERG | Voltage-gated potassium channel subunit Kv11.1 | eag homolog | hERG Potassium Channel 1 | putative potassium channel subunit
Type:
Multi-pass membrane protein
Mol. Mass.:
126672.65
Organism:
Homo sapiens (Human)
Description:
Q12809
Residue:
1159
Sequence:
MPVRRGHVAPQNTFLDTIIRKFEGQSRKFIIANARVENCAVIYCNDGFCELCGYSRAEVMQRPCTCDFLHGPRTQRRAAAQIAQALLGAEERKVEIAFYRKDGSCFLCLVDVVPVKNEDGAVIMFILNFEVVMEKDMVGSPAHDTNHRGPPTSWLAPGRAKTFRLKLPALLALTARESSVRSGGAGGAGAPGAVVVDVDLTPAAPSSESLALDEVTAMDNHVAGLGPAEERRALVGPGSPPRSAPGQLPSPRAHSLNPDASGSSCSLARTRSRESCASVRRASSADDIEAMRAGVLPPPPRHASTGAMHPLRSGLLNSTSDSDLVRYRTISKIPQITLNFVDLKGDPFLASPTSDREIIAPKIKERTHNVTEKVTQVLSLGADVLPEYKLQAPRIHRWTILHYSPFKAVWDWLILLLVIYTAVFTPYSAAFLLKETEEGPPATECGYACQPLAVVDLIVDIMFIVDILINFRTTYVNANEEVVSHPGRIAVHYFKGWFLIDMVAAIPFDLLIFGSGSEELIGLLKTARLLRLVRVARKLDRYSEYGAAVLFLLMCTFALIAHWLACIWYAIGNMEQPHMDSRIGWLHNLGDQIGKPYNSSGLGGPSIKDKYVTALYFTFSSLTSVGFGNVSPNTNSEKIFSICVMLIGSLMYASIFGNVSAIIQRLYSGTARYHTQMLRVREFIRFHQIPNPLRQRLEEYFQHAWSYTNGIDMNAVLKGFPECLQADICLHLNRSLLQHCKPFRGATKGCLRALAMKFKTTHAPPGDTLVHAGDLLTALYFISRGSIEILRGDVVVAILGKNDIFGEPLNLYARPGKSNGDVRALTYCDLHKIHRDDLLEVLDMYPEFSDHFWSSLEITFNLRDTNMIPGSPGSTELEGGFSRQRKRKLSFRRRTDKDTEQPGEVSALGPGRAGAGPSSRGRPGGPWGESPSSGPSSPESSEDEGPGRSSSPLRLVPFSSPRPPGEPPGGEPLMEDCEKSSDTCNPLSGAFSGVSNIFSFWGDSRGRQYQELPRCPAPTPSLLNIPLSSPGRRPRGDVESRLDALQRQLNRLETRLSADMATVLQLLQRQMTLVPPAYSAVTTPGPGPTSTSPLLPVSPLPTLTLDSLSQVSQFMACEELPPGAPELPQEGPTRRLSLPGQLGALTSQPLHRHGSDPGS
Inhibitor
Name:
BDBM50462035
Synonyms:
CHEMBL4228129
Type:
Small organic molecule
Emp. Form.:
C26H30N4O3
Mol. Mass.:
446.5414
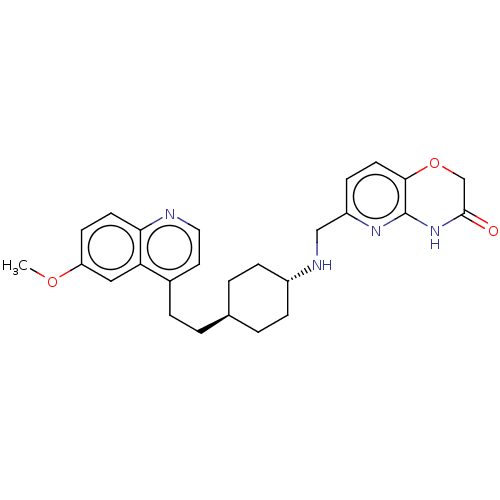
SMILES:
COc1ccc2nccc(CC[C@H]3CC[C@@H](CC3)NCc3ccc4OCC(=O)Nc4n3)c2c1 |r,wU:12.11,wD:15.18,(5.35,-50.76,;5.35,-52.3,;6.68,-53.06,;6.68,-54.61,;8.01,-55.38,;9.34,-54.6,;10.68,-55.37,;12.01,-54.59,;12,-53.04,;10.67,-52.29,;10.66,-50.75,;11.99,-49.97,;13.33,-50.73,;14.65,-49.95,;15.98,-50.73,;15.98,-52.27,;14.66,-53.03,;13.33,-52.28,;17.32,-53.03,;18.65,-52.26,;19.98,-53.03,;19.98,-54.56,;21.31,-55.34,;22.64,-54.55,;23.97,-55.32,;25.3,-54.55,;25.3,-53.01,;26.62,-52.24,;23.96,-52.24,;22.64,-53.01,;21.3,-52.26,;9.34,-53.06,;8.01,-52.3,)|