Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Thiosulfate sulfurtransferase
Ligand
BDBM50523759
Substrate
n/a
Meas. Tech.
ChEMBL_1891102 (CHEMBL4392929)
IC50
>100000±n/a nM
Citation
 Stevens, M; Abdeen, S; Salim, N; Ray, AM; Washburn, A; Chitre, S; Sivinski, J; Park, Y; Hoang, QQ; Chapman, E; Johnson, SM HSP60/10 chaperonin systems are inhibited by a variety of approved drugs, natural products, and known bioactive molecules. Bioorg Med Chem Lett 29:1106-1112 (2019) [PubMed] Article
Stevens, M; Abdeen, S; Salim, N; Ray, AM; Washburn, A; Chitre, S; Sivinski, J; Park, Y; Hoang, QQ; Chapman, E; Johnson, SM HSP60/10 chaperonin systems are inhibited by a variety of approved drugs, natural products, and known bioactive molecules. Bioorg Med Chem Lett 29:1106-1112 (2019) [PubMed] Article More Info.:
Target
Name:
Thiosulfate sulfurtransferase
Synonyms:
2.8.1.1 | Rhodanese | THTR_HUMAN | TST | Thiosulfate sulfurtransferase
Type:
PROTEIN
Mol. Mass.:
33432.06
Organism:
Human
Description:
ChEMBL_118080
Residue:
297
Sequence:
MVHQVLYRALVSTKWLAESIRTGKLGPGLRVLDASWYSPGTREARKEYLERHVPGASFFDIEECRDTASPYEMMLPSEAGFAEYVGRLGISNHTHVVVYDGEHLGSFYAPRVWWMFRVFGHRTVSVLNGGFRNWLKEGHPVTSEPSRPEPAVFKATLDRSLLKTYEQVLENLESKRFQLVDSRSQGRFLGTEPEPDAVGLDSGHIRGAVNMPFMDFLTEDGFEKGPEELRALFQTKKVDLSQPLIATCRKGVTACHVALAAYLCGKPDVAVYDGSWSEWFRRAPPESRVSQGKSEKA
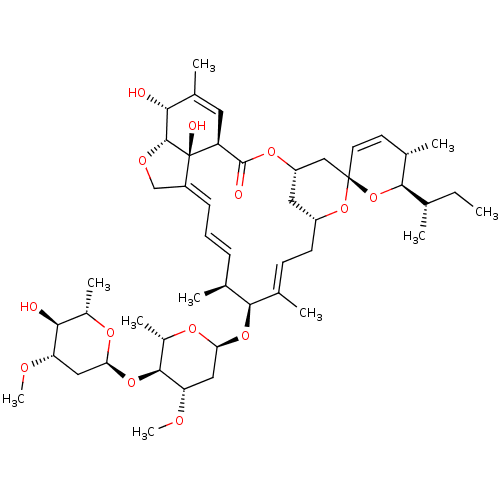
Inhibitor
Name:
BDBM50523759
Synonyms:
Abamectin component b1a | CHEBI:29534
Type:
Small organic molecule
Emp. Form.:
C48H72O14
Mol. Mass.:
873.0769
SMILES:
[H][C@@]12OC\C3=C/C=C/[C@H](C)[C@H](O[C@H]4C[C@H](OC)[C@@H](O[C@H]5C[C@H](OC)[C@@H](O)[C@H](C)O5)[C@H](C)O4)\C(C)=C\C[C@]4([H])C[C@@]([H])(C[C@]5(O4)O[C@]([H])([C@@H](C)CC)[C@@H](C)C=C5)OC(=O)[C@]([H])(C=C(C)[C@H]1O)[C@@]23O |r,c:4,56,t:6,35,64|