Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM291701
Substrate
n/a
Meas. Tech.
ChEMBL_2249035 (CHEMBL5163245)
IC50
29000±n/a nM
Citation
 Huang, Y; Sendzik, M; Zhang, J; Gao, Z; Sun, Y; Wang, L; Gu, J; Zhao, K; Yu, Z; Zhang, L; Zhang, Q; Blanz, J; Chen, Z; Dubost, V; Fang, D; Feng, L; Fu, X; Kiffe, M; Li, L; Luo, F; Luo, X; Mi, Y; Mistry, P; Pearson, D; Piaia, A; Scheufler, C; Terranova, R; Weiss, A; Zeng, J; Zhang, H; Zhang, J; Zhao, M; Dillon, MP; Jeay, S; Qi, W; Moggs, J; Pissot-Soldermann, C; Li, E; Atadja, P; Lingel, A; Oyang, C Discovery of the Clinical Candidate MAK683: An EED-Directed, Allosteric, and Selective PRC2 Inhibitor for the Treatment of Advanced Malignancies. J Med Chem 65:5317-5333 (2022) [PubMed]
Huang, Y; Sendzik, M; Zhang, J; Gao, Z; Sun, Y; Wang, L; Gu, J; Zhao, K; Yu, Z; Zhang, L; Zhang, Q; Blanz, J; Chen, Z; Dubost, V; Fang, D; Feng, L; Fu, X; Kiffe, M; Li, L; Luo, F; Luo, X; Mi, Y; Mistry, P; Pearson, D; Piaia, A; Scheufler, C; Terranova, R; Weiss, A; Zeng, J; Zhang, H; Zhang, J; Zhao, M; Dillon, MP; Jeay, S; Qi, W; Moggs, J; Pissot-Soldermann, C; Li, E; Atadja, P; Lingel, A; Oyang, C Discovery of the Clinical Candidate MAK683: An EED-Directed, Allosteric, and Selective PRC2 Inhibitor for the Treatment of Advanced Malignancies. J Med Chem 65:5317-5333 (2022) [PubMed] More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Human
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
Inhibitor
Name:
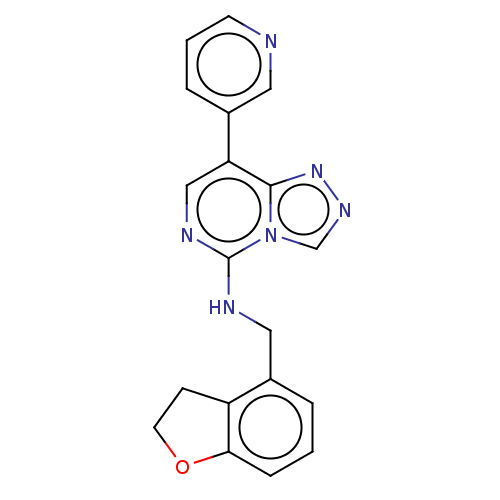
BDBM291701
Synonyms:
N-((2,3-dihydrobenzofuran-4-yl)methyl)-8-(pyridin-3-yl)-[1,2,4]triazolo[4,3-c]pyrimidin-5-amine | US11207325, Example 16 | US9580437, Example 16
Type:
Small organic molecule
Emp. Form.:
C19H16N6O
Mol. Mass.:
344.3699
SMILES:
C(Nc1ncc(-c2cccnc2)c2nncn12)c1cccc2OCCc12