Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase Mer
Ligand
BDBM50444071
Substrate
n/a
Meas. Tech.
ChEMBL_1279519 (CHEMBL3097238)
IC50
3.4±n/a nM
Citation
 Zhang, W; Zhang, D; Stashko, MA; DeRyckere, D; Hunter, D; Kireev, D; Miley, MJ; Cummings, C; Lee, M; Norris-Drouin, J; Stewart, WM; Sather, S; Zhou, Y; Kirkpatrick, G; Machius, M; Janzen, WP; Earp, HS; Graham, DK; Frye, SV; Wang, X Pseudo-cyclization through intramolecular hydrogen bond enables discovery of pyridine substituted pyrimidines as new Mer kinase inhibitors. J Med Chem 56:9683-92 (2014) [PubMed] Article
Zhang, W; Zhang, D; Stashko, MA; DeRyckere, D; Hunter, D; Kireev, D; Miley, MJ; Cummings, C; Lee, M; Norris-Drouin, J; Stewart, WM; Sather, S; Zhou, Y; Kirkpatrick, G; Machius, M; Janzen, WP; Earp, HS; Graham, DK; Frye, SV; Wang, X Pseudo-cyclization through intramolecular hydrogen bond enables discovery of pyridine substituted pyrimidines as new Mer kinase inhibitors. J Med Chem 56:9683-92 (2014) [PubMed] Article More Info.:
Target
Name:
Tyrosine-protein kinase Mer
Synonyms:
MER | MER intracellular domain/EGFR extracellular domain chimera | MERTK | MERTK_HUMAN | Proto-oncogene c-Mer | Proto-oncogene tyrosine-protein kinase MER | Receptor tyrosine kinase MerTK | Tyrosine-protein kinase Mer
Type:
PROTEIN
Mol. Mass.:
110234.77
Organism:
Homo sapiens (Human)
Description:
ChEMBL_1498723
Residue:
999
Sequence:
MGPAPLPLLLGLFLPALWRRAITEAREEAKPYPLFPGPFPGSLQTDHTPLLSLPHASGYQPALMFSPTQPGRPHTGNVAIPQVTSVESKPLPPLAFKHTVGHIILSEHKGVKFNCSISVPNIYQDTTISWWKDGKELLGAHHAITQFYPDDEVTAIIASFSITSVQRSDNGSYICKMKINNEEIVSDPIYIEVQGLPHFTKQPESMNVTRNTAFNLTCQAVGPPEPVNIFWVQNSSRVNEQPEKSPSVLTVPGLTEMAVFSCEAHNDKGLTVSKGVQINIKAIPSPPTEVSIRNSTAHSILISWVPGFDGYSPFRNCSIQVKEADPLSNGSVMIFNTSALPHLYQIKQLQALANYSIGVSCMNEIGWSAVSPWILASTTEGAPSVAPLNVTVFLNESSDNVDIRWMKPPTKQQDGELVGYRISHVWQSAGISKELLEEVGQNGSRARISVQVHNATCTVRIAAVTRGGVGPFSDPVKIFIPAHGWVDYAPSSTPAPGNADPVLIIFGCFCGFILIGLILYISLAIRKRVQETKFGNAFTEEDSELVVNYIAKKSFCRRAIELTLHSLGVSEELQNKLEDVVIDRNLLILGKILGEGEFGSVMEGNLKQEDGTSLKVAVKTMKLDNSSQREIEEFLSEAACMKDFSHPNVIRLLGVCIEMSSQGIPKPMVILPFMKYGDLHTYLLYSRLETGPKHIPLQTLLKFMVDIALGMEYLSNRNFLHRDLAARNCMLRDDMTVCVADFGLSKKIYSGDYYRQGRIAKMPVKWIAIESLADRVYTSKSDVWAFGVTMWEIATRGMTPYPGVQNHEMYDYLLHGHRLKQPEDCLDELYEIMYSCWRTDPLDRPTFSVLRLQLEKLLESLPDVRNQADVIYVNTQLLESSEGLAQGSTLAPLDLNIDPDSIIASCTPRAAISVVTAEVHDSKPHEGRYILNGGSEEWEDLTSAPSAAVTAEKNSVLPGERLVRNGVSWSHSSMLPLGSSLPDELLFADDSSEGSEVLM
Inhibitor
Name:
BDBM50444071
Synonyms:
CHEMBL3092794
Type:
Small organic molecule
Emp. Form.:
C26H39N7O2
Mol. Mass.:
481.6336
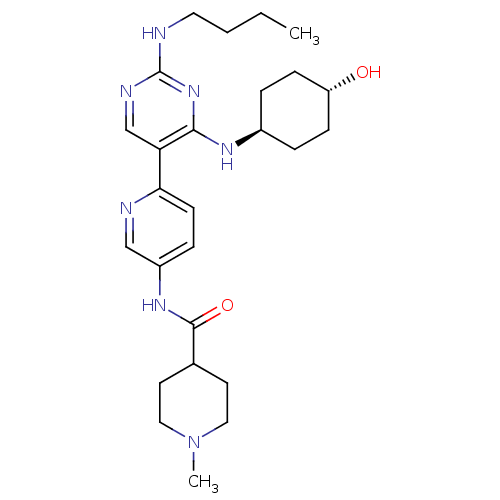
SMILES:
CCCCNc1ncc(c(N[C@H]2CC[C@H](O)CC2)n1)-c1ccc(NC(=O)C2CCN(C)CC2)cn1 |r,wU:11.10,wD:14.14,(41.44,-46.25,;42.77,-45.48,;44.11,-46.25,;45.44,-45.48,;46.77,-46.25,;48.11,-45.48,;49.44,-46.25,;50.78,-45.48,;50.78,-43.93,;49.44,-43.17,;49.44,-41.63,;48.1,-40.86,;46.77,-41.63,;45.44,-40.86,;45.44,-39.32,;44.1,-38.54,;46.77,-38.55,;48.1,-39.31,;48.11,-43.94,;52.11,-43.16,;53.44,-43.93,;54.77,-43.15,;54.76,-41.61,;56.09,-40.83,;57.43,-41.6,;57.44,-43.14,;58.76,-40.82,;60.09,-41.59,;61.42,-40.82,;61.42,-39.28,;62.75,-38.51,;60.08,-38.51,;58.74,-39.29,;53.42,-40.85,;52.09,-41.62,)|