Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Amyloid-beta precursor protein
Ligand
BDBM50084980
Substrate
n/a
Meas. Tech.
ChEMBL_1545148 (CHEMBL3750502)
IC50
33000±n/a nM
Citation
More Info.:
Target
Name:
Amyloid-beta precursor protein
Synonyms:
A4 | A4_HUMAN | ABPP | AD1 | AICD-50 | AICD-57 | AICD-59 | AID(50) | AID(57) | AID(59) | APP | APPI | Alzheimer disease amyloid protein | Amyloid beta A4 protein | Amyloid beta Protein | Amyloid beta protein (sAPPbeta) | Amyloid beta protein Abeta(1-42) | Amyloid intracellular domain 50 | Amyloid intracellular domain 57 | Amyloid intracellular domain 59 | Amyloid protein (Abeta42b) | Amyloid ╬▓-protein (A╬▓42) | Beta amyloid A4 protein | Beta-APP40 | Beta-APP42 | Beta-amyloid protein 40 | Beta-amyloid protein 42 | C31 | C83 | C99 | CVAP | Cerebral vascular amyloid peptide | Gamma Secretase | Gamma-CTF(50) | Gamma-CTF(57) | Gamma-CTF(59) | Gamma-secretase | Gamma-secretase C-terminal fragment 50 | Gamma-secretase C-terminal fragment 57 | Gamma-secretase C-terminal fragment 59 | P3(40) | P3(42) | PN-II | PreA4 | Protease nexin-II | S-APP-alpha | S-APP-beta | Soluble APP-alpha | Soluble APP-beta | beta-Amyloid Precursor Protein (APP)
Type:
Single-pass type I membrane protein
Mol. Mass.:
86890.41
Organism:
Homo sapiens (Human)
Description:
P05067
Residue:
770
Sequence:
MLPGLALLLLAAWTARALEVPTDGNAGLLAEPQIAMFCGRLNMHMNVQNGKWDSDPSGTKTCIDTKEGILQYCQEVYPELQITNVVEANQPVTIQNWCKRGRKQCKTHPHFVIPYRCLVGEFVSDALLVPDKCKFLHQERMDVCETHLHWHTVAKETCSEKSTNLHDYGMLLPCGIDKFRGVEFVCCPLAEESDNVDSADAEEDDSDVWWGGADTDYADGSEDKVVEVAEEEEVAEVEEEEADDDEDDEDGDEVEEEAEEPYEEATERTTSIATTTTTTTESVEEVVREVCSEQAETGPCRAMISRWYFDVTEGKCAPFFYGGCGGNRNNFDTEEYCMAVCGSAMSQSLLKTTQEPLARDPVKLPTTAASTPDAVDKYLETPGDENEHAHFQKAKERLEAKHRERMSQVMREWEEAERQAKNLPKADKKAVIQHFQEKVESLEQEAANERQQLVETHMARVEAMLNDRRRLALENYITALQAVPPRPRHVFNMLKKYVRAEQKDRQHTLKHFEHVRMVDPKKAAQIRSQVMTHLRVIYERMNQSLSLLYNVPAVAEEIQDEVDELLQKEQNYSDDVLANMISEPRISYGNDALMPSLTETKTTVELLPVNGEFSLDDLQPWHSFGADSVPANTENEVEPVDARPAADRGLTTRPGSGLTNIKTEEISEVKMDAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIATVIVITLVMLKKKQYTSIHHGVVEVDAAVTPEERHLSKMQQNGYENPTYKFFEQMQN
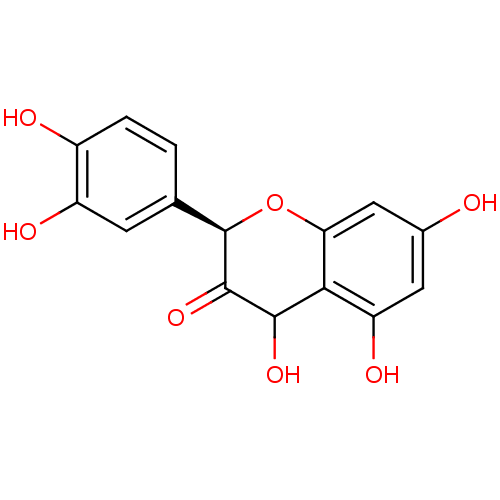
Inhibitor
Name:
BDBM50084980
Synonyms:
(+)-taxifolin | (-)-Epicatechol | (2R,3R)-(-)-Epicatechin | (2R,3R)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one | (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-2H-chromene-3,5,7-triol(2R,3R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-2,3-dihydro-4H-chromen-4-one | (2R,3R)-dihydroquercetin | (2R-trans)-2-(3,4-dihydroxyphenyl)-2,3-dihydro-3,5,7-trihydroxy-4-benzopyrone | CHEMBL66 | L-Epicatechin
Type:
Small organic molecule
Emp. Form.:
C15H12O7
Mol. Mass.:
304.2516
SMILES:
OC1C(=O)[C@H](Oc2cc(O)cc(O)c12)c1ccc(O)c(O)c1 |r|