Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Urotensin-2 receptor
Ligand
BDBM50112107
Substrate
n/a
Meas. Tech.
ChEMBL_210769 (CHEMBL815740)
EC50
1.8±n/a nM
Citation
 Flohr, S; Kurz, M; Kostenis, E; Brkovich, A; Fournier, A; Klabunde, T Identification of nonpeptidic urotensin II receptor antagonists by virtual screening based on a pharmacophore model derived from structure-activity relationships and nuclear magnetic resonance studies on urotensin II. J Med Chem 45:1799-805 (2002) [PubMed] Article
Flohr, S; Kurz, M; Kostenis, E; Brkovich, A; Fournier, A; Klabunde, T Identification of nonpeptidic urotensin II receptor antagonists by virtual screening based on a pharmacophore model derived from structure-activity relationships and nuclear magnetic resonance studies on urotensin II. J Med Chem 45:1799-805 (2002) [PubMed] Article More Info.:
Target
Name:
Urotensin-2 receptor
Synonyms:
G-protein coupled receptor 14 | GPR14 | UR-II-R | UR2R_HUMAN | UTS2R | Urotensin II receptor | Urotensin-II
Type:
Enzyme Catalytic Domain
Mol. Mass.:
42159.71
Organism:
Homo sapiens (Human)
Description:
Urotensin-II UTS2R HUMAN::Q9UKP6
Residue:
389
Sequence:
MALTPESPSSFPGLAATGSSVPEPPGGPNATLNSSWASPTEPSSLEDLVATGTIGTLLSAMGVVGVVGNAYTLVVTCRSLRAVASMYVYVVNLALADLLYLLSIPFIVATYVTKEWHFGDVGCRVLFGLDFLTMHASIFTLTVMSSERYAAVLRPLDTVQRPKGYRKLLALGTWLLALLLTLPVMLAMRLVRRGPKSLCLPAWGPRAHRAYLTLLFATSIAGPGLLIGLLYARLARAYRRSQRASFKRARRPGARALRLVLGIVLLFWACFLPFWLWQLLAQYHQAPLAPRTARIVNYLTTCLTYGNSCANPFLYTLLTRNYRDHLRGRVRGPGSGGGRGPVPSLQPRARFQRCSGRSLSSCSPQPTDSLVLAPAAPARPAPEGPRAPA
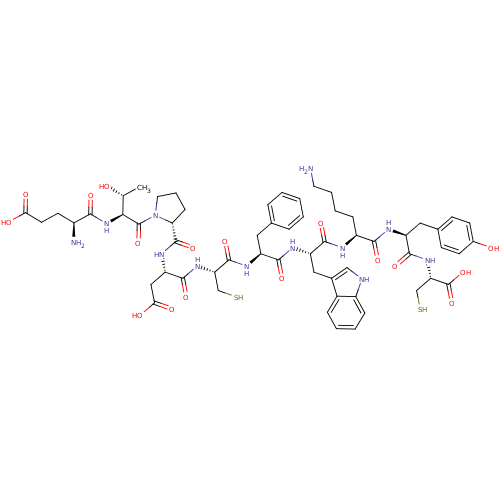
Inhibitor
Name:
BDBM50112107
Synonyms:
CHEMBL385616 | Glu-Thr-Pro-Asp-Cys-Phe-Trp-Lys-Tyr-Cys
Type:
Small organic molecule
Emp. Form.:
C59H78N12O17S2
Mol. Mass.:
1291.451
SMILES:
C[C@@H](O)[C@H](NC(=O)[C@@H](N)CCC(O)=O)C(=O)N1CCC[C@@H]1C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CS)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CS)C(O)=O