Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Receptor-type tyrosine-protein phosphatase beta
Ligand
BDBM359121
Substrate
n/a
Meas. Tech.
Inhibitory Activity to HPTP-β
IC50
0.080±n/a nM
Citation
 Peters, K Methods of treating intraocular pressure with activators of Tie-2 US Patent US11666558 Publication Date 6/6/2023
Peters, K Methods of treating intraocular pressure with activators of Tie-2 US Patent US11666558 Publication Date 6/6/2023 More Info.:
Target
Name:
Receptor-type tyrosine-protein phosphatase beta
Synonyms:
PTPB | PTPRB | PTPRB_HUMAN | Protein-tyrosine phosphatase beta | R-PTP-beta | Receptor-type tyrosine-protein phosphatase beta | Receptor-type tyrosine-protein phosphatase beta (PTPβ) | VE-PTP | Vascular endothelial protein tyrosine phosphatase
Type:
Protein
Mol. Mass.:
224324.80
Organism:
Homo sapiens (Human)
Description:
P23467
Residue:
1997
Sequence:
MLSHGAGLALWITLSLLQTGLAEPERCNFTLAESKASSHSVSIQWRILGSPCNFSLIYSSDTLGAALCPTFRIDNTTYGCNLQDLQAGTIYNFRIISLDEERTVVLQTDPLPPARFGVSKEKTTSTSLHVWWTPSSGKVTSYEVQLFDENNQKIQGVQIQESTSWNEYTFFNLTAGSKYNIAITAVSGGKRSFSVYTNGSTVPSPVKDIGISTKANSLLISWSHGSGNVERYRLMLMDKGILVHGGVVDKHATSYAFHGLTPGYLYNLTVMTEAAGLQNYRWKLVRTAPMEVSNLKVTNDGSLTSLKVKWQRPPGNVDSYNITLSHKGTIKESRVLAPWITETHFKELVPGRLYQVTVSCVSGELSAQKMAVGRTFPDKVANLEANNNGRMRSLVVSWSPPAGDWEQYRILLFNDSVVLLNITVGKEETQYVMDDTGLVPGRQYEVEVIVESGNLKNSERCQGRTVPLAVLQLRVKHANETSLSIMWQTPVAEWEKYIISLADRDLLLIHKSLSKDAKEFTFTDLVPGRKYMATVTSISGDLKNSSSVKGRTVPAQVTDLHVANQGMTSSLFTNWTQAQGDVEFYQVLLIHENVVIKNESISSETSRYSFHSLKSGSLYSVVVTTVSGGISSRQVVVEGRTVPSSVSGVTVNNSGRNDYLSVSWLLAPGDVDNYEVTLSHDGKVVQSLVIAKSVRECSFSSLTPGRLYTVTITTRSGKYENHSFSQERTVPDKVQGVSVSNSARSDYLRVSWVHATGDFDHYEVTIKNKNNFIQTKSIPKSENECVFVQLVPGRLYSVTVTTKSGQYEANEQGNGRTIPEPVKDLTLRNRSTEDLHVTWSGANGDVDQYEIQLLFNDMKVFPPFHLVNTATEYRFTSLTPGRQYKILVLTISGDVQQSAFIEGFTVPSAVKNIHISPNGATDSLTVNWTPGGGDVDSYTVSAFRHSQKVDSQTIPKHVFEHTFHRLEAGEQYQIMIASVSGSLKNQINVVGRTVPASVQGVIADNAYSSYSLIVSWQKAAGVAERYDILLLTENGILLRNTSEPATTKQHKFEDLTPGKKYKIQILTVSGGLFSKEAQTEGRTVPAAVTDLRITENSTRHLSFRWTASEGELSWYNIFLYNPDGNLQERAQVDPLVQSFSFQNLLQGRMYKMVIVTHSGELSNESFIFGRTVPASVSHLRGSNRNTTDSLWFNWSPASGDFDFYELILYNPNGTKKENWKDKDLTEWRFQGLVPGRKYVLWVVTHSGDLSNKVTAESRTAPSPPSLMSFADIANTSLAITWKGPPDWTDYNDFELQWLPRDALTVFNPYNNRKSEGRIVYGLRPGRSYQFNVKTVSGDSWKTYSKPIFGSVRTKPDKIQNLHCRPQNSTAIACSWIPPDSDFDGYSIECRKMDTQEVEFSRKLEKEKSLLNIMMLVPHKRYLVSIKVQSAGMTSEVVEDSTITMIDRPPPPPPHIRVNEKDVLISKSSINFTVNCSWFSDTNGAVKYFTVVVREADGSDELKPEQQHPLPSYLEYRHNASIRVYQTNYFASKCAENPNSNSKSFNIKLGAEMESLGGKCDPTQQKFCDGPLKPHTAYRISIRAFTQLFDEDLKEFTKPLYSDTFFSLPITTESEPLFGAIEGVSAGLFLIGMLVAVVALLICRQKVSHGRERPSARLSIRRDRPLSVHLNLGQKGNRKTSCPIKINQFEGHFMKLQADSNYLLSKEYEELKDVGRNQSCDIALLPENRGKNRYNNILPYDATRVKLSNVDDDPCSDYINASYIPGNNFRREYIVTQGPLPGTKDDFWKMVWEQNVHNIVMVTQCVEKGRVKCDHYWPADQDSLYYGDLILQMLSESVLPEWTIREFKICGEEQLDAHRLIRHFHYTVWPDHGVPETTQSLIQFVRTVRDYINRSPGAGPTVVHCSAGVGRTGTFIALDRILQQLDSKDSVDIYGAVHDLRLHRVHMVQTECQYVYLHQCVRDVLRARKLRSEQENPLFPIYENVNPEYHRDPVYSRH
Inhibitor
Name:
BDBM359121
Synonyms:
US10220048, Compound AA31 | US10952992, No. AA31 | US11413242, Compound AA31 | US11666558, Example AA31
Type:
Small organic molecule
Emp. Form.:
C29H29ClN4O8S3
Mol. Mass.:
693.211
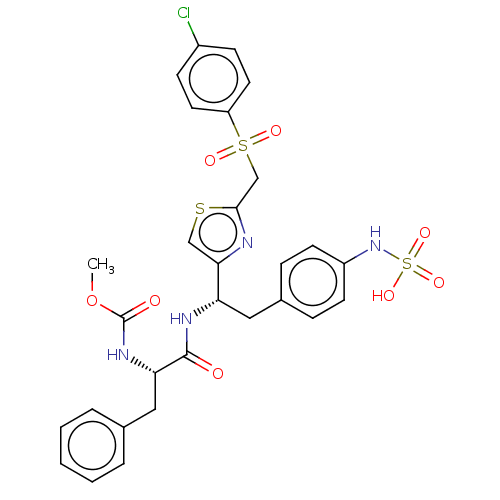
SMILES:
COC(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccc(NS(O)(=O)=O)cc1)c1csc(CS(=O)(=O)c2ccc(Cl)cc2)n1 |r|