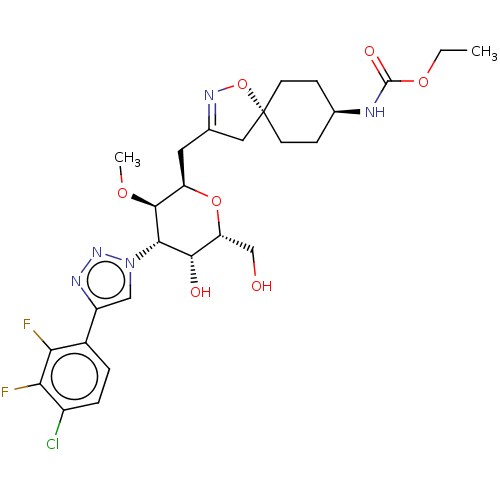
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Galectin-3
Ligand
BDBM620361
Substrate
n/a
Meas. Tech.
Evaluation of Compound Inhibitory Activity (IC50)
IC50
19.0±n/a nM
Citation
 BOLLLI, M; GATFIELD, J; GRISOSTOMI, C; REMEN, L; SAGER, C; ZUMBRUNN, C SPIRO DERIVATIVES OF ALPHA-D-GALACTOPYRANOSIDES US Patent US20230295182 Publication Date 9/21/2023
BOLLLI, M; GATFIELD, J; GRISOSTOMI, C; REMEN, L; SAGER, C; ZUMBRUNN, C SPIRO DERIVATIVES OF ALPHA-D-GALACTOPYRANOSIDES US Patent US20230295182 Publication Date 9/21/2023 More Info.:
Target
Name:
Galectin-3
Synonyms:
LEG3_HUMAN | LGALS3 | MAC2
Type:
Enzyme
Mol. Mass.:
26156.54
Organism:
Homo sapiens (Human)
Description:
P17931
Residue:
250
Sequence:
MADNFSLHDALSGSGNPNPQGWPGAWGNQPAGAGGYPGASYPGAYPGQAPPGAYPGQAPPGAYPGAPGAYPGAPAPGVYPGPPSGPGAYPSSGQPSATGAYPATGPYGAPAGPLIVPYNLPLPGGVVPRMLITILGTVKPNANRIALDFQRGNDVAFHFNPRFNENNRRVIVCNTKLDNNWGREERQSVFPFESGKPFKIQVLVEPDHFKVAVNDAHLLQYNHRVKKLNEISKLGISGDIDLTSASYTMI
Inhibitor
Name:
BDBM620361
Synonyms:
US20230295182, Example 2.11 | ethyl ((5r,8R)-3-(((2R,3R,4S,5R,6R)-4-(4-(4-chloro-2,3-difluorophenyl)-1H- 1,2,3-triazol-1-yl)-5-hydroxy-6-(hydroxymethyl)-3-methoxytetrahydro-2H-pyran- 2-yl)methyl)-1-oxa-2-azaspiro[4.5]dec-2-en-8-yl)carbamate
Type:
Small organic molecule
Emp. Form.:
C27H34ClF2N5O7
Mol. Mass.:
614.038
SMILES:
CCOC(=O)N[C@H]1CC[C@@]2(CC(C[C@H]3O[C@H](CO)[C@H](O)[C@@H]([C@H]3OC)n3cc(nn3)-c3ccc(Cl)c(F)c3F)=NO2)CC1 |wU:9.42,21.22,13.12,wD:15.15,18.18,20.24,6.5,c:40,(9.26,-1.95,;7.93,-2.72,;6.6,-1.95,;5.26,-2.72,;5.26,-4.26,;3.93,-1.95,;2.59,-2.72,;1.26,-1.95,;-.07,-2.72,;-.07,-4.26,;-1.48,-3.64,;-2.51,-4.78,;-4.04,-4.62,;-4.67,-3.21,;-6.2,-3.05,;-6.83,-1.65,;-8.36,-1.48,;-9.26,-2.73,;-5.92,-.4,;-6.55,1.01,;-4.39,-.56,;-3.76,-1.97,;-2.23,-2.13,;-1.33,-.88,;-3.48,.69,;-1.94,.69,;-1.47,2.15,;-2.71,3.06,;-3.96,2.15,;-0,2.63,;1.14,1.6,;2.61,2.07,;2.93,3.58,;4.39,4.05,;1.78,4.61,;2.1,6.11,;.32,4.13,;-.83,5.16,;-1.74,-6.11,;-.23,-5.79,;1.26,-5.03,;2.59,-4.26,)|