Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Thromboxane-A synthase
Ligand
BDBM10033
Substrate
BDBM10042
Meas. Tech.
Aromatase Assay
IC50
1230±n/a nM
Citation
 Jacobs, C; Frotscher, M; Dannhardt, G; Hartmann, RW 1-imidazolyl(alkyl)-substituted di- and tetrahydroquinolines and analogues: syntheses and evaluation of dual inhibitors of thromboxane A(2) synthase and aromatase. J Med Chem 43:1841-51 (2000) [PubMed] Article
Jacobs, C; Frotscher, M; Dannhardt, G; Hartmann, RW 1-imidazolyl(alkyl)-substituted di- and tetrahydroquinolines and analogues: syntheses and evaluation of dual inhibitors of thromboxane A(2) synthase and aromatase. J Med Chem 43:1841-51 (2000) [PubMed] Article More Info.:
Target
Name:
Thromboxane-A synthase
Synonyms:
CYP5 | CYP5A1 | Cytochrome P450 5A1 | P450 TxA2 | TBXAS1 | THAS_HUMAN | TXA synthase | TXAS | TXS | Thromboxane A2 Synthase | Thromboxane A2 Synthase (P450 TxA2) | Thromboxane Alpha | Thromboxane prostanoid | Thromboxane synthase | Thromboxane-A synthase
Type:
Enzyme
Mol. Mass.:
60524.67
Organism:
Homo sapiens (Human)
Description:
P24557
Residue:
533
Sequence:
MEALGFLKLEVNGPMVTVALSVALLALLKWYSTSAFSRLEKLGLRHPKPSPFIGNLTFFRQGFWESQMELRKLYGPLCGYYLGRRMFIVISEPDMIKQVLVENFSNFTNRMASGLEFKSVADSVLFLRDKRWEEVRGALMSAFSPEKLNEMVPLISQACDLLLAHLKRYAESGDAFDIQRCYCNYTTDVVASVAFGTPVDSWQAPEDPFVKHCKRFFEFCIPRPILVLLLSFPSIMVPLARILPNKNRDELNGFFNKLIRNVIALRDQQAAEERRRDFLQMVLDARHSASPMGVQDFDIVRDVFSSTGCKPNPSRQHQPSPMARPLTVDEIVGQAFIFLIAGYEIITNTLSFATYLLATNPDCQEKLLREVDVFKEKHMAPEFCSLEEGLPYLDMVIAETLRMYPPAFRFTREAAQDCEVLGQRIPAGAVLEMAVGALHHDPEHWPSPETFNPERFTAEARQQHRPFTYLPFGAGPRSCLGVRLGLLEVKLTLLHVLHKFRFQACPETQVPLQLESKSALGPKNGVYIKIVSR
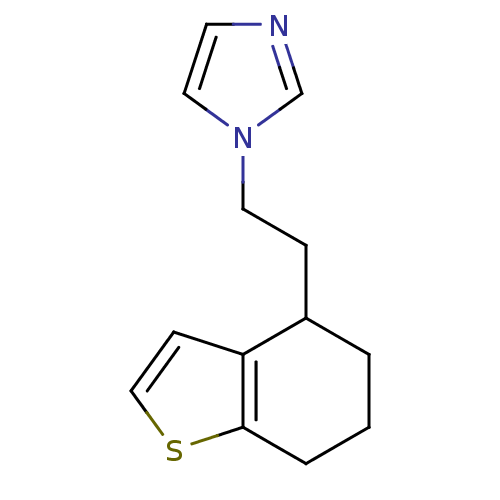
Inhibitor
Name:
BDBM10033
Synonyms:
1-[2-(4,5,6,7-Tetrahydrobenzo[b]thiophen-4-yl)ethyl]-1H-imidazole | 1-[2-(4,5,6,7-tetrahydro-1-benzothiophen-4-yl)ethyl]-1H-imidazole | tetrahydrobenzo[b]thiophene 28
Type:
Small organic molecule
Emp. Form.:
C13H16N2S
Mol. Mass.:
232.345
SMILES:
C(Cn1ccnc1)C1CCCc2sccc12
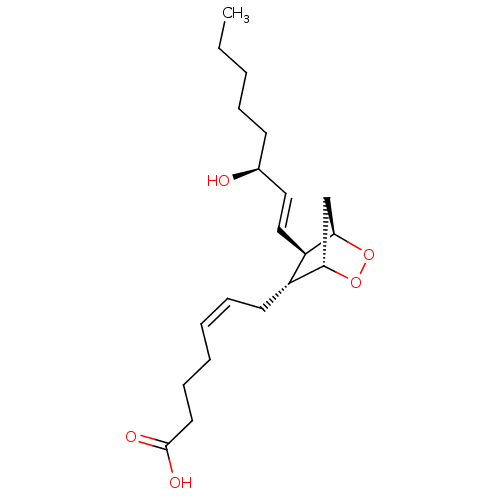
Substrate
Name:
BDBM10042
Synonyms:
(5Z)-7-[(1R,4S,5R,6R)-6-[(1E,3S)-3-hydroxyoct-1-en-1-yl]-2,3-dioxabicyclo[2.2.1]heptan-5-yl]hept-5-enoic acid | (5Z,13E,15S)-9alpha, 11alpha-epidioxy-15-hydroxyprosta-5,13-dienoic acid | 9,11-epoxymethano PGH2 | PGH2 | Prostaglandin H2
Type:
Small organic molecule
Emp. Form.:
C20H32O5
Mol. Mass.:
352.4651
SMILES:
CCCCC[C@H](O)\C=C\[C@H]1[C@H]2C[C@H](OO2)[C@@H]1C\C=C/CCCC(O)=O