Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Urokinase-type plasminogen activator
Ligand
BDBM14152
Substrate
BDBM14484
Meas. Tech.
Enzyme Assay and Determination of the Inhibition Constants
Ki
14±n/a nM
Citation
 Katz, BA; Luong, C; Ho, JD; Somoza, JR; Gjerstad, E; Tang, J; Williams, SR; Verner, E; Mackman, RL; Young, WB; Sprengeler, PA; Chan, H; Mortara, K; Janc, JW; McGrath, ME Dissecting and designing inhibitor selectivity determinants at the S1 site using an artificial Ala190 protease (Ala190 uPA). J Mol Biol 344:527-47 (2004) [PubMed] Article
Katz, BA; Luong, C; Ho, JD; Somoza, JR; Gjerstad, E; Tang, J; Williams, SR; Verner, E; Mackman, RL; Young, WB; Sprengeler, PA; Chan, H; Mortara, K; Janc, JW; McGrath, ME Dissecting and designing inhibitor selectivity determinants at the S1 site using an artificial Ala190 protease (Ala190 uPA). J Mol Biol 344:527-47 (2004) [PubMed] Article More Info.:
Target
Name:
Urokinase-type plasminogen activator
Synonyms:
3.4.21.73 | PLAU | U-plasminogen activator | UROK_HUMAN | Urokinase | Urokinase-type plasminogen activator (uPA) | Urokinase-type plasminogen activator chain B | Urokinase-type plasminogen activator long chain A | Urokinase-type plasminogen activator short chain A | Urokinase-type plasminogen activator/surface receptor | uPA
Type:
Enzyme
Mol. Mass.:
48528.62
Organism:
Homo sapiens (Human)
Description:
P00749
Residue:
431
Sequence:
MRALLARLLLCVLVVSDSKGSNELHQVPSNCDCLNGGTCVSNKYFSNIHWCNCPKKFGGQHCEIDKSKTCYEGNGHFYRGKASTDTMGRPCLPWNSATVLQQTYHAHRSDALQLGLGKHNYCRNPDNRRRPWCYVQVGLKLLVQECMVHDCADGKKPSSPPEELKFQCGQKTLRPRFKIIGGEFTTIENQPWFAAIYRRHRGGSVTYVCGGSLISPCWVISATHCFIDYPKKEDYIVYLGRSRLNSNTQGEMKFEVENLILHKDYSADTLAHHNDIALLKIRSKEGRCAQPSRTIQTICLPSMYNDPQFGTSCEITGFGKENSTDYLYPEQLKMTVVKLISHRECQQPHYYGSEVTTKMLCAADPQWKTDSCQGDSGGPLVCSLQGRMTLTGIVSWGRGCALKDKPGVYTRVSHFLPWIRSHTKEENGLAL
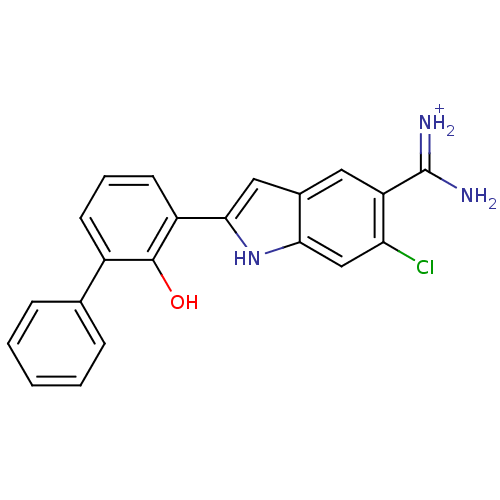
Inhibitor
Name:
BDBM14152
Synonyms:
6-CHLORO-2-(2-HYDROXY-BIPHENYL-3-YL)-1H-INDOLE-5-CARBOXAMIDINE | APC-10302 | CA-14 | CRA-10302 | {amino[6-chloro-2-(2-hydroxy-3-phenylphenyl)-1H-indol-5-yl]methylidene}azanium
Type:
Small organic molecule
Emp. Form.:
C21H17ClN3O
Mol. Mass.:
362.832
SMILES:
NC(=[NH2+])c1cc2cc([nH]c2cc1Cl)-c1cccc(-c2ccccc2)c1O