Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Major prion protein
Ligand
BDBM357271
Substrate
n/a
Meas. Tech.
Human Platelet Aggregation Inhibition Test
IC50
20.0±n/a nM
Citation
 Tran, T; Ibarra, JB; Shin, Y; Ullman, B; Zou, N; Zeng, X Pyrazolyl substituted carbonic acid derivatives as modulators of the prostacyclin (PGI2) receptor useful for the treatment of disorders related thereto US Patent US10214518 Publication Date 2/26/2019
Tran, T; Ibarra, JB; Shin, Y; Ullman, B; Zou, N; Zeng, X Pyrazolyl substituted carbonic acid derivatives as modulators of the prostacyclin (PGI2) receptor useful for the treatment of disorders related thereto US Patent US10214518 Publication Date 2/26/2019 More Info.:
Target
Name:
Major prion protein
Synonyms:
ALTPRP | ASCR | CD_antigen=CD230 | PRIO_HUMAN | PRIP | PRNP | PRP | PrP27-30 | PrP33-35C | Prion protein | major prion protein preproprotein
Type:
PROTEIN
Mol. Mass.:
27671.97
Organism:
Homo sapiens (Human)
Description:
ChEMBL_158887
Residue:
253
Sequence:
MANLGCWMLVLFVATWSDLGLCKKRPKPGGWNTGGSRYPGQGSPGGNRYPPQGGGGWGQPHGGGWGQPHGGGWGQPHGGGWGQPHGGGWGQGGGTHSQWNKPSKPKTNMKHMAGAAAAGAVVGGLGGYMLGSAMSRPIIHFGSDYEDRYYRENMHRYPNQVYYRPMDEYSNQNNFVHDCVNITIKQHTVTTTTKGENFTETDVKMMERVVEQMCITQYERESQAYYQRGSSMVLFSSPPVILLISFLIFLIVG
Inhibitor
Name:
BDBM357271
Synonyms:
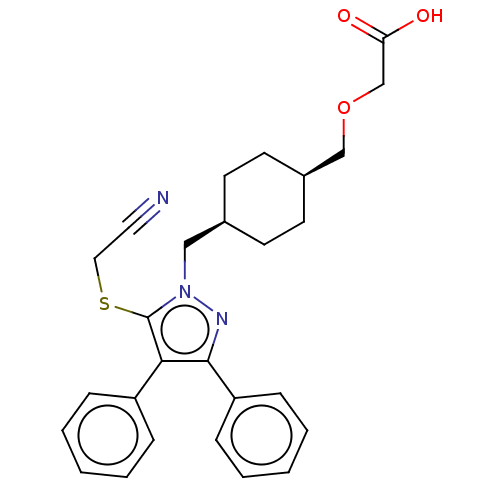
2-(((1s,4s)-4-((5- (cyanomethylthio)-3,4- diphenyl-1H-pyrazol-1- yl)methyl)cyclohexyl) methoxy)acetic acid | US10214518, Compound 184 | US11098034, Compound 184
Type:
Small organic molecule
Emp. Form.:
C27H29N3O3S
Mol. Mass.:
475.602
SMILES:
OC(=O)COC[C@H]1CC[C@@H](Cn2nc(c(c2SCC#N)-c2ccccc2)-c2ccccc2)CC1 |r,wU:9.9,6.5,(9.46,-2.31,;8.13,-1.54,;8.13,0,;6.79,-2.31,;5.46,-1.54,;4.13,-2.31,;2.79,-1.54,;1.46,-2.31,;.13,-1.54,;.13,0,;-1.21,.77,;-2.54,0,;-2.77,-1.52,;-4.29,-1.78,;-5,-.41,;-3.92,.69,;-4.32,2.18,;-3.23,3.26,;-3.63,4.75,;-4.03,6.24,;-6.49,-.01,;-6.89,1.48,;-8.37,1.87,;-9.46,.79,;-9.06,-.7,;-7.58,-1.1,;-4.69,-3.26,;-6.17,-3.66,;-6.57,-5.15,;-5.48,-6.24,;-4,-5.84,;-3.6,-4.35,;1.46,.77,;2.79,0,)|