Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Adenosylhomocysteine nucleosidase
Ligand
BDBM22112
Substrate
BDBM22111
Meas. Tech.
MTAP/MTAN Inhibition Assay
pH
7±n/a
Temperature
295.15±n/a K
Ki
84±6 nM
Km
23000±n/a nM
Citation
 Evans, GB; Furneaux, RH; Greatrex, B; Murkin, AS; Schramm, VL; Tyler, PC Azetidine based transition state analogue inhibitors of N-ribosyl hydrolases and phosphorylases. J Med Chem 51:948-56 (2008) [PubMed] Article
Evans, GB; Furneaux, RH; Greatrex, B; Murkin, AS; Schramm, VL; Tyler, PC Azetidine based transition state analogue inhibitors of N-ribosyl hydrolases and phosphorylases. J Med Chem 51:948-56 (2008) [PubMed] Article More Info.:
Target
Name:
Adenosylhomocysteine nucleosidase
Synonyms:
5 -methylthioadenosine / S-adenosylhomocysteine nucleosidase | Methylthioadenosine Nucleosidase(MTAN)
Type:
Enzyme
Mol. Mass.:
24666.14
Organism:
Streptococcus pneumoniae
Description:
Q8DQ16
Residue:
230
Sequence:
MKIGIIAAMPEELAYLVQHLDNTQEQVVLGNTYHTGTIASHEVVLVESGIGKVMSAMSVAILADHFQVDALINTGSAGAVAEGIAVGDVVIADKLAYHDVDVTAFGYAYGQMAQQPLYFESDKTFVAQIQESLSQLDQNWHLGLIATGDSFVAGNDKIEAIKSHFPEVLAVEMEGAAIAQAAHTLNLPVLVIRAMSDNANHEANIFFDEFIIEAGRRSAQVLLAFLKALD
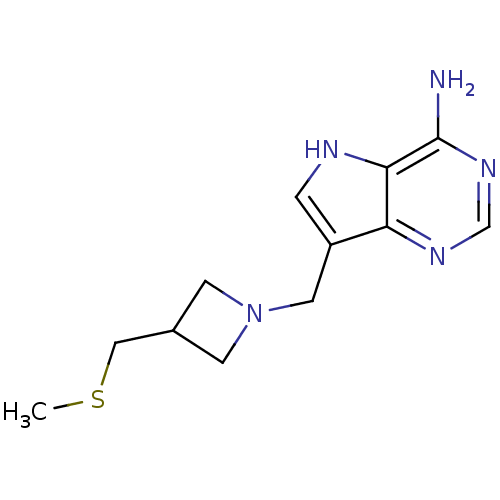
Inhibitor
Name:
BDBM22112
Synonyms:
7-({3-[(methylsulfanyl)methyl]azetidin-1-yl}methyl)-5H-pyrrolo[3,2-d]pyrimidin-4-amine | Azetidine based compound, 44
Type:
Small organic molecule
Emp. Form.:
C12H17N5S
Mol. Mass.:
263.362
SMILES:
CSCC1CN(Cc2c[nH]c3c(N)ncnc23)C1
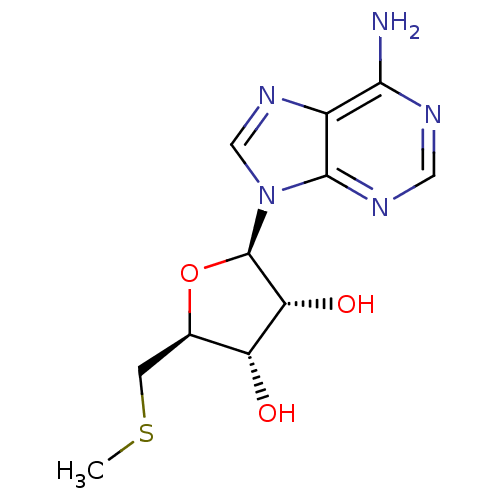
Substrate
Name:
BDBM22111
Synonyms:
(2R,3R,4S,5S)-2-(6-amino-9H-purin-9-yl)-5-[(methylsulfanyl)methyl]oxolane-3,4-diol | (2R,3R,4S,5S)-2-(6-aminopurin-9-yl)-5-(methylsulfanylmethyl)oxolane-3,4-diol | 5'-Methylthioado | 5-methylthioadenosine | CHEMBL277041 | MTA
Type:
Nucleoside or nucleotide
Emp. Form.:
C11H15N5O3S
Mol. Mass.:
297.334
SMILES:
CSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)ncnc12