Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
fMet-Leu-Phe receptor
Ligand
BDBM377265
Substrate
n/a
Meas. Tech.
Measuring Agonistic Activity of Compounds in hFPR1-Gα15-CHO and hFPR2-Aq-CHO Cell
EC50
500±n/a nM
Citation
 Jakob, F; Nordhoff, S; Rider, D; Wagener, M; Bahrenberg, G; Dunkern, T 6-membered cyclic amines or lactames substituted with urea and phenyl US Patent US10265310 Publication Date 4/23/2019
Jakob, F; Nordhoff, S; Rider, D; Wagener, M; Bahrenberg, G; Dunkern, T 6-membered cyclic amines or lactames substituted with urea and phenyl US Patent US10265310 Publication Date 4/23/2019 More Info.:
Target
Name:
fMet-Leu-Phe receptor
Synonyms:
FPR | FPR1 | FPR1_HUMAN | Formyl peptide Receptor | N-formyl peptide receptor 1 | N-formylpeptide chemoattractant receptor | fMLP receptor | fMet-Leu-Phe receptor | formyl peptide receptor 1
Type:
Enzyme Catalytic Domain
Mol. Mass.:
38456.14
Organism:
Homo sapiens (Human)
Description:
gi_4503779
Residue:
350
Sequence:
METNSSLPTNISGGTPAVSAGYLFLDIITYLVFAVTFVLGVLGNGLVIWVAGFRMTHTVTTISYLNLAVADFCFTSTLPFFMVRKAMGGHWPFGWFLCKFVFTIVDINLFGSVFLIALIALDRCVCVLHPVWTQNHRTVSLAKKVIIGPWVMALLLTLPVIIRVTTVPGKTGTVACTFNFSPWTNDPKERINVAVAMLTVRGIIRFIIGFSAPMSIVAVSYGLIATKIHKQGLIKSSRPLRVLSFVAAAFFLCWSPYQVVALIATVRIRELLQGMYKEIGIAVDVTSALAFFNSCLNPMLYVFMGQDFRERLIHALPASLERALTEDSTQTSDTATNSTLPSAEVELQAK
Inhibitor
Name:
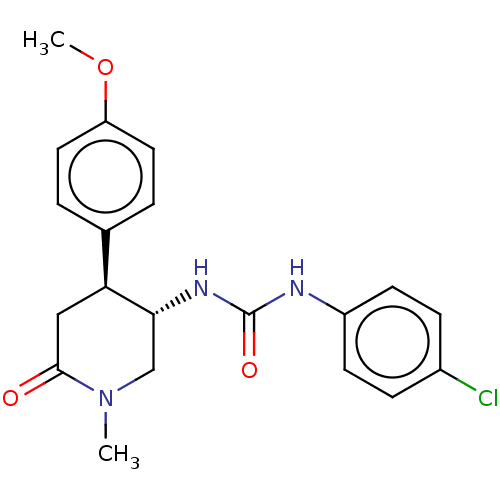
BDBM377265
Synonyms:
US10265310, Example 11 | trans-ent2-(4-chlorophenyl)-3-(4-(4-methoxyphenyl)-1-methyl-6-oxopiperidin-3-yl)urea (Enantiomer 2)
Type:
Small organic molecule
Emp. Form.:
C20H22ClN3O3
Mol. Mass.:
387.86
SMILES:
COc1ccc(cc1)[C@@H]1CC(=O)N(C)C[C@H]1NC(=O)Nc1ccc(Cl)cc1