Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A1
Ligand
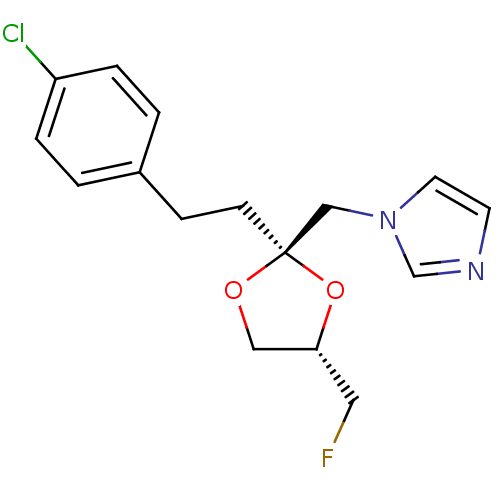
BDBM31682
Substrate
erythromycin
IC50
4000±n/a nM
Citation
 Vlahakis, JZ; Hum, M; Rahman, MN; Jia, Z; Nakatsu, K; Szarek, WA Synthesis and evaluation of imidazole-dioxolane compounds as selective heme oxygenase inhibitors: effect of substituents at the 4-position of the dioxolane ring. Bioorg Med Chem 17:2461-75 (2009) [PubMed] Article
Vlahakis, JZ; Hum, M; Rahman, MN; Jia, Z; Nakatsu, K; Szarek, WA Synthesis and evaluation of imidazole-dioxolane compounds as selective heme oxygenase inhibitors: effect of substituents at the 4-position of the dioxolane ring. Bioorg Med Chem 17:2461-75 (2009) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A1
Synonyms:
CP3A1_RAT | CYPIIIA1 | Cyp3a-1 | Cyp3a1 | P450-PCN1
Type:
Enzyme
Mol. Mass.:
57928.74
Organism:
Rattus norvegicus (rat)
Description:
CYP3A1/3A2 prepared from rat liver microsomes was used in enzyme assays.
Residue:
504
Sequence:
MDLLSALTLETWVLLAVVLVLLYGFGTRTHGLFKKQGIPGPKPLPFFGTVLNYYMGLWKFDVECHKKYGKIWGLFDGQMPLFAITDTEMIKNVLVKECFSVFTNRRDFGPVGIMGKAVSVAKDEEWKRYRALLSPTFTSGRLKEMFPIIEQYGDILVKYLKQEAETGKPVTMKKVFGAYSMDVITSTSFGVNVDSLNNPKDPFVEKTKKLLRFDFFDPLFLSVVLFPFLTPIYEMLNICMFPKDSIEFFKKFVYRMKETRLDSVQKHRVDFLQLMMNAHNDSKDKESHTALSDMEITAQSIIFIFAGYEPTSSTLSFVLHSLATHPDTQKKLQEEIDRALPNKAPPTYDTVMEMEYLDMVLNETLRLYPIGNRLERVCKKDVEINGVFMPKGSVVMIPSYALHRDPQHWPEPEEFRPERFSKENKGSIDPYVYLPFGNGPRNCIGMRFALMNMKLALTKVLQNFSFQPCKETQIPLKLSRQGLLQPTKPIILKVVPRDEIITGS
Inhibitor
Name:
BDBM31682
Synonyms:
imidazole-dioxolane, 32
Type:
Small organic molecule
Emp. Form.:
C16H18ClFN2O2
Mol. Mass.:
324.778
SMILES:
FC[C@@H]1CO[C@@](CCc2ccc(Cl)cc2)(Cn2ccnc2)O1 |r|
Substrate
Name:
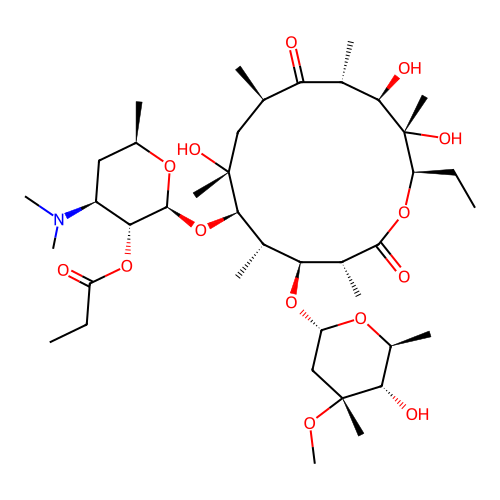
BDBM31688
Synonyms:
Erythromycin Estolate
Type:
macrolide antibiotic
Emp. Form.:
C52H97NO18S
Mol. Mass.:
1056.387
SMILES:
CCCCCCCCCCCCOS(O)(=O)=O.CC[C@H]1OC(=O)[C@H](C)[C@@H](O[C@H]2C[C@@](C)(OC)[C@@H](O)[C@H](C)O2)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2OC(=O)CC)N(C)C)[C@](C)(O)C[C@@H](C)C(=O)[C@H](C)[C@@H](O)[C@]1(C)O