Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Splicing factor 3B subunit 3
Ligand
BDBM36361
Substrate
n/a
Meas. Tech.
WiDr
pH
7.8±0
Temperature
277.15±0 K
EC50
70.2±0.0 nM
Citation
 Kotake, Y; Sagane, K; Owa, T; Mimori-Kiyosue, Y; Shimizu, H; Uesugi, M; Ishihama, Y; Iwata, M; Mizui, Y Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol 3:570-5 (2007) [PubMed] Article
Kotake, Y; Sagane, K; Owa, T; Mimori-Kiyosue, Y; Shimizu, H; Uesugi, M; Ishihama, Y; Iwata, M; Mizui, Y Splicing factor SF3b as a target of the antitumor natural product pladienolide. Nat Chem Biol 3:570-5 (2007) [PubMed] Article More Info.:
Target
Name:
Splicing factor 3B subunit 3
Synonyms:
KIAA0017 | SAP130 | SF3B3 | SF3B3_HUMAN
Type:
PROTEIN
Mol. Mass.:
135544.53
Organism:
Homo sapiens (Human)
Description:
Q15393
Residue:
1217
Sequence:
MFLYNLTLQRATGISFAIHGNFSGTKQQEIVVSRGKILELLRPDPNTGKVHTLLTVEVFGVIRSLMAFRLTGGTKDYIVVGSDSGRIVILEYQPSKNMFEKIHQETFGKSGCRRIVPGQFLAVDPKGRAVMISAIEKQKLVYILNRDAAARLTISSPLEAHKANTLVYHVVGVDVGFENPMFACLEMDYEEADNDPTGEAAANTQQTLTFYELDLGLNHVVRKYSEPLEEHGNFLITVPGGSDGPSGVLICSENYITYKNFGDQPDIRCPIPRRRNDLDDPERGMIFVCSATHKTKSMFFFLAQTEQGDIFKITLETDEDMVTEIRLKYFDTVPVAAAMCVLKTGFLFVASEFGNHYLYQIAHLGDDDEEPEFSSAMPLEEGDTFFFQPRPLKNLVLVDELDSLSPILFCQIADLANEDTPQLYVACGRGPRSSLRVLRHGLEVSEMAVSELPGNPNAVWTVRRHIEDEFDAYIIVSFVNATLVLSIGETVEEVTDSGFLGTTPTLSCSLLGDDALVQVYPDGIRHIRADKRVNEWKTPGKKTIVKCAVNQRQVVIALTGGELVYFEMDPSGQLNEYTERKEMSADVVCMSLANVPPGEQRSRFLAVGLVDNTVRIISLDPSDCLQPLSMQALPAQPESLCIVEMGGTEKQDELGERGSIGFLYLNIGLQNGVLLRTVLDPVTGDLSDTRTRYLGSRPVKLFRVRMQGQEAVLAMSSRSWLSYSYQSRFHLTPLSYETLEFASGFASEQCPEGIVAISTNTLRILALEKLGAVFNQVAFPLQYTPRKFVIHPESNNLIIIETDHNAYTEATKAQRKQQMAEEMVEAAGEDERELAAEMAAAFLNENLPESIFGAPKAGNGQWASVIRVMNPIQGNTLDLVQLEQNEAAFSVAVCRFSNTGEDWYVLVGVAKDLILNPRSVAGGFVYTYKLVNNGEKLEFLHKTPVEEVPAAIAPFQGRVLIGVGKLLRVYDLGKKKLLRKCENKHIANYISGIQTIGHRVIVSDVQESFIWVRYKRNENQLIIFADDTYPRWVTTASLLDYDTVAGADKFGNICVVRLPPNTNDEVDEDPTGNKALWDRGLLNGASQKAEVIMNYHVGETVLSLQKTTLIPGGSESLVYTTLSGGIGILVPFTSHEDHDFFQHVEMHLRSEHPPLCGRDHLSFRSYYFPVKNVIDGDLCEQFNSMEPNKQKNVSEELDRTPPEVSKKLEDIRTRYAF
Inhibitor
Name:
BDBM36361
Synonyms:
CID16202134 | Pladienolide analog 9
Type:
Small organic molecule
Emp. Form.:
C51H77BF2N4O11
Mol. Mass.:
970.985
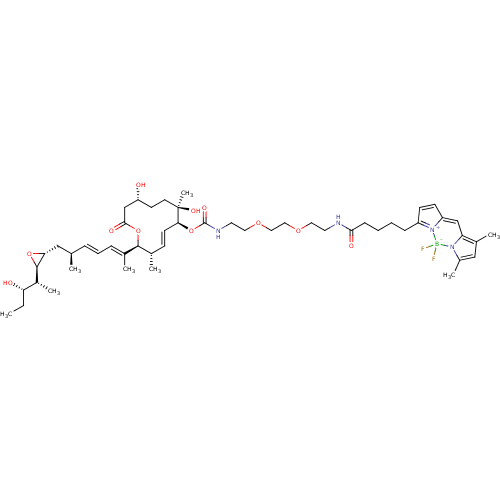
SMILES:
CC[C@H](O)[C@@H](C)[C@H]1O[C@@H]1C[C@H](C)\C=C\C=C(/C)[C@H]1OC(=O)C[C@H](O)CC[C@@](C)(O)[C@@H](OC(=O)NCCOCCOCCNC(=O)CCCCC2=[N+]3C(C=C2)=Cc2c(C)cc(C)n2[B-]3(F)F)\C=C\[C@@H]1C |c:53,55,t:50,69|