Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
1,3-beta-glucan synthase
Ligand
BDBM50478216
Substrate
n/a
Meas. Tech.
ChEMBL_731129 (CHEMBL1697535)
IC50
69.8±n/a nM
Citation
 Garcia-Effron, G; Lee, S; Park, S; Cleary, JD; Perlin, DS Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690-9 (2009) [PubMed] Article
Garcia-Effron, G; Lee, S; Park, S; Cleary, JD; Perlin, DS Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-beta-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob Agents Chemother 53:3690-9 (2009) [PubMed] Article More Info.:
Target
Name:
1,3-beta-glucan synthase
Synonyms:
1,3-beta-glucan synthase component GSC2 | Beta-1,3-glucan synthase catalytic subunit | FKS2
Type:
PROTEIN
Mol. Mass.:
217615.31
Organism:
Candida glabrata
Description:
ChEMBL_18077
Residue:
1897
Sequence:
MSYDQGGNGNWQNTDPNGNYYYNGAENNEFYDQDYASQQPEQQQGGEGYYDEYGQPNYNYMNDPQQGQMPQQQPGGYDNDGYYDSYYNNQMNAGVGNGLGPDQTNFSDFSSYGPPPFQNNQANYTPSQLSYSNNGMGSNGMNMSGSSTPVYGNYDPNAIAMTLPNDPYPAWTADPQSPVSIEQIEDVFIDLTNKFGFQRDSMRNIFDLFMTLLDSRTSRMSPDQALLSVHADYIGGDTANYKKWYFAAQLDMDDEVGFRNMNLGKLSRKARKAKKKNKKAMEEANPEDAAEVLNKIEGDNSLEASDFRWKTKMNMLTPIERVRQVALYMLIWGEANQVRFTSECLCFIYKCASDYLESPLCQQRTEPIPEGDYLNRVITPIYQFIRNQVYEIVDGRYVKREKDHNKIIGYDDVNQLFWYPEGITKIVLEDGTKLTDIPSEERYLRLGEVAWNDVFFKTYKETRTWLHLVTNFNRIWIMHVSVYWMYVAYNSPTFYTHNYQQLVNNQPVPAYRWASAALAGTVASAIQLFATVCEWWFVPRKWAGAQHLSRRFWFLCGILGVNLGPLIFVFAYEKDTVQSKAGHAVAAVTFFIAVATVLFFSIMPLGGLFTSYMQKSSRRYVASQTFTASFAPLQGLDRWLSYLVWVTVFAAKYSESYFFLILSLRDPIRILSTTTMRCTGEYWWGSKLCRHQSKIVLGFMIATDFILFFLDTYLWYIVVNTVFSVGKSFYLGISILTPWRNIFTRLPKRIYSKILATTDMEIKYKPKVLISQIWNAIIISMYREHLLAIDHVQKLLYHQVPSEIEGKRTLRAPTFFVSQDDNNFETEFFPRNSEAERRISFFAQSLATPMPEPLPVDNMPTFTVLTPHYSERILLSLREIIREDDQFSRVTLLEYLKQLHPVEWECFVKDTKILAEETAAYENEEPQDPEKSDALKTQIDDLPFYCIGFKSAAPEYTLRTRIWASLRSQTLYRTVSGFMNYARAIKLLYRVENPEIVQMFGGNAEGLERELEKMARRKFKFLVSMQRLAKFKPHELENTEFLLRAYPDLQIAYLDEEPPLNEGEEPRIYSALIDGHCEMLENGRRRPKFRVQLSGNPILGDGKSDNQNHALIFYRGEYIQLIDANQDNYLEECLKIRSVLAEFEELNAEPVYPYTPGVKYEDQKTNHPVAIVGAREYIFSENSGVLGDVAAGKEQTFGTLFARTLAQIGGKLHYGHPDFINATFMTTRSGLSKAQKGLHLNEDIYAGMNALLRGGRIKHCEYYQCGKGRDLGFGTILNFTTKIGAGMGEQMLSREYYYLGTQLPVDRFLTFYYAHPGFHLNNLFIQLSLQMFMLTLVNLHALAHESILCIYDRNKPKTDVLYPIGCYNFSPAIDWIRRYTLSIFIVFWIAFVPIVVQELIERGLWKATQRFFRHILSLSPMFEVFAGQIYSAALLSDMTVGGARYISTGRGFATSRIPFSILYSRFASSAIYMGARSMLMLLFGTVAHWQAPLLWFWASLSALLFSPFIFNPHQFSWEDFFLDYRDYIRWLSRGNNKYHKNSWIGYVRMARSRITGFKRKLIGDDSEKAAGDANRAHRTNLILAELIPTAINAGSCFIGFTFINAQTGVKATDDDRVNSVLRVVLCTLGPIAVDVGVLFFCLGMSCCSGPLFGMCCKKTGAVMAAVAHGVSVVIHIGFFIVMWVLEGFNFTRMLVGVATVIQCQRFIFQLMTILLLTREFKNDHANTAFWTGKWYGSGFGYMAWTQPMRELTAKVIEMSEFAADFVLGHVILFAQFPVLCIPAIDKFHSIMLFWLKPSRHIRPPIYSLKQSRLRKRMVKRYLTLYIIIFLVFAGAIVGPAVAASHVPQDIGHTLTGPFHNIVQPRNKSNNDTGLQISTYSNHYYTHTPSLKTWSTIK
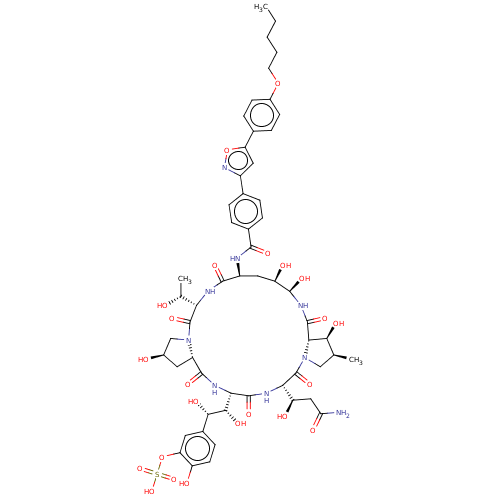
Inhibitor
Name:
BDBM50478216
Synonyms:
CHEBI:600520 | Micafungin | Mycamine
Type:
Small organic molecule
Emp. Form.:
C56H71N9O23S
Mol. Mass.:
1270.274
SMILES:
[H][C@@]12C[C@@H](O)CN1C(=O)[C@@]([H])(NC(=O)[C@H](C[C@@H](O)[C@@H](O)NC(=O)[C@]1([H])[C@@H](O)[C@@H](C)CN1C(=O)[C@@]([H])(NC(=O)[C@@]([H])(NC2=O)[C@H](O)[C@@H](O)c1ccc(O)c(OS(O)(=O)=O)c1)[C@H](O)CC(N)=O)NC(=O)c1ccc(cc1)-c1cc(on1)-c1ccc(OCCCCC)cc1)[C@@H](C)O