Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Maltase-glucoamylase
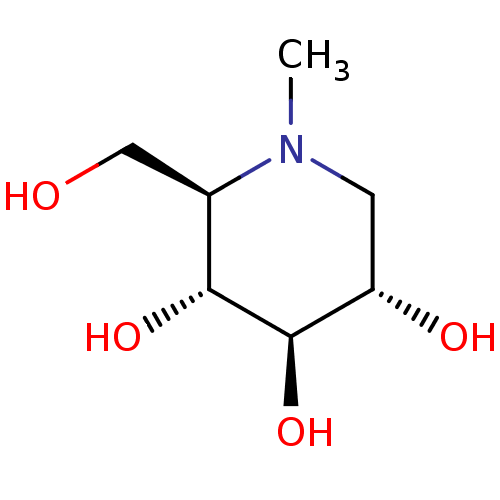
Ligand
BDBM18353
Substrate
n/a
Meas. Tech.
ChEMBL_101864 (CHEMBL710107)
IC50
120±n/a nM
Citation
 Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem 38:2349-56 (1995) [PubMed] Article
Asano, N; Kizu, H; Oseki, K; Tomioka, E; Matsui, K; Okamoto, M; Baba, M N-alkylated nitrogen-in-the-ring sugars: conformational basis of inhibition of glycosidases and HIV-1 replication. J Med Chem 38:2349-56 (1995) [PubMed] Article More Info.:
Target
Name:
Maltase-glucoamylase
Synonyms:
Alpha glucosidase | Alpha-1,4-glucosidase | Glucan 1,4-alpha-glucosidase | MGA | MGAM | MGAML | MGA_HUMAN | Maltase | Maltase-glucoamylase, intestinal | Synonyms=MGA
Type:
Enzyme
Mol. Mass.:
209817.06
Organism:
Homo sapiens (Human)
Description:
O43451
Residue:
2753
Sequence:
MARKKLKKFTTLEIVLSVLLLVLFIISIVLIVLLAKESLKSTAPDPGTTGTPDPGTTGTPDPGTTGTTHARTTGPPDPGTTGTTPVSAECPVVNELERINCIPDQPPTKATCDQRGCCWNPQGAVSVPWCYYSKNHSYHVEGNLVNTNAGFTARLKNLPSSPVFGSNVDNVLLTAEYQTSNRFHFKLTDQTNNRFEVPHEHVQSFSGNAAASLTYQVEISRQPFSIKVTRRSNNRVLFDSSIGPLLFADQFLQLSTRLPSTNVYGLGEHVHQQYRHDMNWKTWPIFNRDTTPNGNGTNLYGAQTFFLCLEDASGLSFGVFLMNSNAMEVVLQPAPAITYRTIGGILDFYVFLGNTPEQVVQEYLELIGRPALPSYWALGFHLSRYEYGTLDNMREVVERNRAAQLPYDVQHADIDYMDERRDFTYDSVDFKGFPEFVNELHNNGQKLVIIVDPAISNNSSSSKPYGPYDRGSDMKIWVNSSDGVTPLIGEVWPGQTVFPDYTNPNCAVWWTKEFELFHNQVEFDGIWIDMNEVSNFVDGSVSGCSTNNLNNPPFTPRILDGYLFCKTLCMDAVQHWGKQYDIHNLYGYSMAVATAEAAKTVFPNKRSFILTRSTFAGSGKFAAHWLGDNTATWDDLRWSIPGVLEFNLFGIPMVGPDICGFALDTPEELCRRWMQLGAFYPFSRNHNGQGYKDQDPASFGADSLLLNSSRHYLNIRYTLLPYLYTLFFRAHSRGDTVARPLLHEFYEDNSTWDVHQQFLWGPGLLITPVLDEGAEKVMAYVPDAVWYDYETGSQVRWRKQKVEMELPGDKIGLHLRGGYIFPTQQPNTTTLASRKNPLGLIIALDENKEAKGELFWDNGETKDTVANKVYLLCEFSVTQNRLEVNISQSTYKDPNNLAFNEIKILGTEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDIDLLLGEAYTVEWSIKIRDEEKIDCYPDENGASAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVYANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNKNRYEVPVPLNIPSMPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFSDMFIRISTRLPSKYLYGFGETEHRSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEIASLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPHLESRDRGLSSKTLCMESQQILPDGSLVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDAAFVNISRNVLQTRYTLLPYLYTLMQKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRKNPLGLIIALDENKEAKGELFWDDGQTKDTVAKKVYLLCEFSVTQNHLEVTISQSTYKDPNNLAFNEIKILGMEEPSNVTVKHNGVPSQTSPTVTYDSNLKVAIITDINLFLGEAYTVEWSIKIRDEEKIDCYPDENGDSAENCTARGCIWEASNSSGVPFCYFVNDLYSVSDVQYNSHGATADISLKSSVHANAFPSTPVNPLRLDVTYHKNEMLQFKIYDPNNNRYEVPVPLNIPSVPSSTPEGQLYDVLIKKNPFGIEIRRKSTGTIIWDSQLLGFTFNDMFIRISTRLPSKYLYGFGETEHTSYRRDLEWHTWGMFSRDQPPGYKKNSYGVHPYYMGLEEDGSAHGVLLLNSNAMDVTFQPLPALTYRTTGGVLDFYVFLGPTPELVTQQYTELIGRPVMVPYWSLGFQLCRYGYQNDSEISSLYDEMVAAQIPYDVQYSDIDYMERQLDFTLSPKFAGFPALINRMKADGMRVILILDPAISGNETQPYPAFTRGVEDDVFIKYPNDGDIVWGKVWPDFPDVVVNGSLDWDSQVELYRAYVAFPDFFRNSTAKWWKREIEELYNNPQNPERSLKFDGMWIDMNEPSSFVNGAVSPGCRDASLNHPPYMPYLESRDRGLSSKTLCMESQQILPDGSPVQHYNVHNLYGWSQTRPTYEAVQEVTGQRGVVITRSTFPSSGRWAGHWLGDNTAAWDQLKKSIIGMMEFSLFGISYTGADICGFFQDAEYEMCVRWMQLGAFYPFSRNHNTIGTRRQDPVSWDVAFVNISRTVLQTRYTLLPYLYTLMHKAHTEGVTVVRPLLHEFVSDQVTWDIDSQFLLGPAFLVSPVLERNARNVTAYFPRARWYDYYTGVDINARGEWKTLPAPLDHINLHVRGGYILPWQEPALNTHLSRQKFMGFKIALDDEGTAGGWLFWDDGQSIDTYGKGLYYLASFSASQNTMQSHIIFNNYITGTNPLKLGYIEIWGVGSVPVTSVSISVSGMVITPSFNNDPTTQVLSIDVTDRNISLHNFTSLTWISTL