Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Cytochrome P450 3A4
Ligand
BDBM50044425
Substrate
n/a
Meas. Tech.
ChEBML_223457
Ki
33±n/a nM
Citation
 Walker, KA; Kertesz, DJ; Rotstein, DM; Swinney, DC; Berry, PW; So, OY; Webb, AS; Watson, DM; Mak, AY; Burton, PM Selective inhibition of mammalian lanosterol 14 alpha-demethylase: a possible strategy for cholesterol lowering. J Med Chem 36:2235-7 (1993) [PubMed] Article
Walker, KA; Kertesz, DJ; Rotstein, DM; Swinney, DC; Berry, PW; So, OY; Webb, AS; Watson, DM; Mak, AY; Burton, PM Selective inhibition of mammalian lanosterol 14 alpha-demethylase: a possible strategy for cholesterol lowering. J Med Chem 36:2235-7 (1993) [PubMed] Article More Info.:
Target
Name:
Cytochrome P450 3A4
Synonyms:
Albendazole monooxygenase | Albendazole sulfoxidase | CP3A4_HUMAN | CYP3A3 | CYP3A4 | CYPIIIA3 | CYPIIIA4 | Cytochrome P450 3A3 | Cytochrome P450 3A4 (CYP3A4) | Cytochrome P450 HLp | Nifedipine oxidase | Quinine 3-monooxygenase | Taurochenodeoxycholate 6-alpha-hydroxylase
Type:
Enzyme
Mol. Mass.:
57349.57
Organism:
Homo sapiens (Human)
Description:
n/a
Residue:
503
Sequence:
MALIPDLAMETWLLLAVSLVLLYLYGTHSHGLFKKLGIPGPTPLPFLGNILSYHKGFCMFDMECHKKYGKVWGFYDGQQPVLAITDPDMIKTVLVKECYSVFTNRRPFGPVGFMKSAISIAEDEEWKRLRSLLSPTFTSGKLKEMVPIIAQYGDVLVRNLRREAETGKPVTLKDVFGAYSMDVITSTSFGVNIDSLNNPQDPFVENTKKLLRFDFLDPFFLSITVFPFLIPILEVLNICVFPREVTNFLRKSVKRMKESRLEDTQKHRVDFLQLMIDSQNSKETESHKALSDLELVAQSIIFIFAGYETTSSVLSFIMYELATHPDVQQKLQEEIDAVLPNKAPPTYDTVLQMEYLDMVVNETLRLFPIAMRLERVCKKDVEINGMFIPKGVVVMIPSYALHRDPKYWTEPEKFLPERFSKKNKDNIDPYIYTPFGSGPRNCIGMRFALMNMKLALIRVLQNFSFKPCKETQIPLKLSLGGLLQPEKPVVLKVESRDGTVSGA
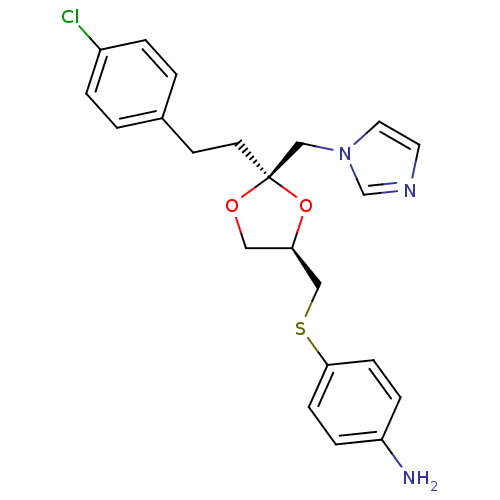
Inhibitor
Name:
BDBM50044425
Synonyms:
4-{(2R,4R)-2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazol-1-ylmethyl-[1,3]dioxolan-4-ylmethylsulfanyl}-phenylamine | 4-{2-[2-(4-Chloro-phenyl)-ethyl]-2-imidazol-1-ylmethyl-[1,3]dioxolan-4-ylmethylsulfanyl}-phenylamine | CHEMBL305220
Type:
Small organic molecule
Emp. Form.:
C22H24ClN3O2S
Mol. Mass.:
429.963
SMILES:
Nc1ccc(SC[C@H]2CO[C@@](CCc3ccc(Cl)cc3)(Cn3ccnc3)O2)cc1