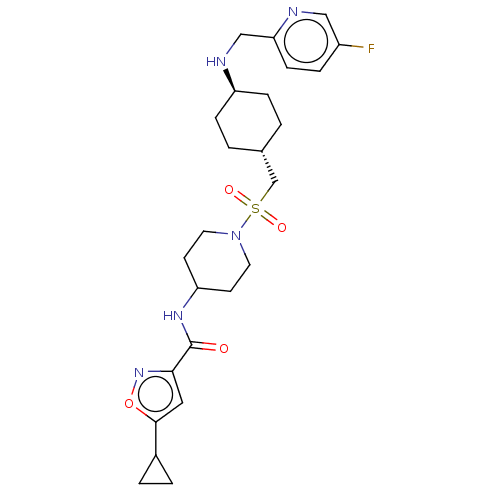
Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Histone-lysine N-methyltransferase SMYD3
Ligand
BDBM50509610
Substrate
n/a
Meas. Tech.
ChEMBL_1838817 (CHEMBL4338950)
IC50
<1.000000±n/a nM
Citation
 Su, DS; Qu, J; Schulz, M; Blackledge, CW; Yu, H; Zeng, J; Burgess, J; Reif, A; Stern, M; Nagarajan, R; Pappalardi, MB; Wong, K; Graves, AP; Bonnette, W; Wang, L; Elkins, P; Knapp-Reed, B; Carson, JD; McHugh, C; Mohammad, H; Kruger, R; Luengo, J; Heerding, DA; Creasy, CL Discovery of Isoxazole Amides as Potent and Selective SMYD3 Inhibitors. ACS Med Chem Lett 11:133-140 (2020) [PubMed] Article
Su, DS; Qu, J; Schulz, M; Blackledge, CW; Yu, H; Zeng, J; Burgess, J; Reif, A; Stern, M; Nagarajan, R; Pappalardi, MB; Wong, K; Graves, AP; Bonnette, W; Wang, L; Elkins, P; Knapp-Reed, B; Carson, JD; McHugh, C; Mohammad, H; Kruger, R; Luengo, J; Heerding, DA; Creasy, CL Discovery of Isoxazole Amides as Potent and Selective SMYD3 Inhibitors. ACS Med Chem Lett 11:133-140 (2020) [PubMed] Article More Info.:
Target
Name:
Histone-lysine N-methyltransferase SMYD3
Synonyms:
SET and MYND domain-containing protein 3 | SMYD3 | SMYD3_HUMAN | ZMYND1 | ZNFN3A1 | Zinc finger MYND domain-containing protein 1
Type:
Enzyme
Mol. Mass.:
49101.22
Organism:
Homo sapiens (Human)
Description:
Q9H7B4-2
Residue:
428
Sequence:
MEPLKVEKFATAKRGNGLRAVTPLRPGELLFRSDPLAYTVCKGSRGVVCDRCLLGKEKLMRCSQCRVAKYCSAKCQKKAWPDHKRECKCLKSCKPRYPPDSVRLLGRVVFKLMDGAPSESEKLYSFYDLESNINKLTEDKKEGLRQLVMTFQHFMREEIQDASQLPPAFDLFEAFAKVICNSFTICNAEMQEVGVGLYPSISLLNHSCDPNCSIVFNGPHLLLRAVRDIEVGEELTICYLDMLMTSEERRKQLRDQYCFECDCFRCQTQDKDADMLTGDEQVWKEVQESLKKIEELKAHWKWEQVLAMCQAIISSNSERLPDINIYQLKVLDCAMDACINLGLLEEALFYGTRTMEPYRIFFPGSHPVRGVQVMKVGKLQLHQGMFPQAMKNLRLAFDIMRVTHGREHSLIEDLILLLEECDANIRAS
Inhibitor
Name:
BDBM50509610
Synonyms:
CHEMBL4460507
Type:
Small organic molecule
Emp. Form.:
C25H34FN5O4S
Mol. Mass.:
519.632
SMILES:
Fc1ccc(CN[C@H]2CC[C@H](CS(=O)(=O)N3CCC(CC3)NC(=O)c3cc(on3)C3CC3)CC2)nc1 |r,wU:7.6,wD:10.10,(20.89,-9.64,;20.9,-8.1,;19.58,-7.32,;19.59,-5.79,;20.93,-5.05,;20.94,-3.51,;19.62,-2.73,;18.28,-3.49,;18.27,-5.03,;16.94,-5.79,;15.6,-5.01,;14.27,-5.78,;12.93,-4.99,;12.15,-3.65,;13.7,-3.65,;11.6,-5.77,;10.26,-4.99,;8.94,-5.77,;8.94,-7.31,;10.26,-8.07,;11.6,-7.31,;7.61,-8.08,;6.27,-7.31,;6.27,-5.77,;4.94,-8.08,;4.79,-9.61,;3.28,-9.93,;2.5,-8.61,;3.53,-7.46,;2.66,-11.34,;1.41,-12.25,;2.82,-12.88,;15.61,-3.47,;16.95,-2.72,;22.25,-5.81,;22.24,-7.34,)|