Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
T-type calcium channel alpha 1G subunit
Ligand
BDBM50514579
Substrate
n/a
Meas. Tech.
ChEMBL_1857572 (CHEMBL4358301)
EC50
3800±n/a nM
Citation
More Info.:
Target
Name:
T-type calcium channel alpha 1G subunit
Synonyms:
Cacna1g | T-type calcium channel alpha 1G subunit
Type:
PROTEIN
Mol. Mass.:
250746.67
Organism:
Mus musculus
Description:
ChEMBL_118081
Residue:
2265
Sequence:
MDEEEDGAGAEESGQPRSFTQLNDLSGAGGRQGPGSTEKDPGSADSEAEGLPYPALAPVVFFYLSQDSRPRSWCLRTVCNPWFERVSMLVILLNCVTLGMFRPCEDIACDSQRCRILQAFDDFIFAFFAVEMVVKMVALGIFGKKCYLGDTWNRLDFFIVIAGMLEYSLDLQNVSFSAVRTVRVLRPLRAINRVPSMRILVTLLLDTLPMLGNVLLLCFFVFFIFGIVGVQLWAGLLRNRCFLPENFSLPLSVDLEPYYQTENEDESPFICSQPRENGMRSCRSVPTLRGEGGGGPPCGLDYEAYNSSSNTTCVNWNQYYTNCSAGEHNPFKGAINFDNIGYAWIAIFQVITLEGWVDIMYFVMDAHSFYNFIYFILLIIVGSFFMINLCLVVIATQFSETKQRESQLMREQRVRFLSNASTLASFSEPGSCYEELLKYLVYILRKAARRLAQVSRAVGVRAGLLSSPVVRGGQEPQPSGSCSRSHRRLSVHHLVHHHHHHHHHYHLGNGTLRVPRASPEIQDRDANGSRWLMLPPPSTPTPSGGPPRGAESVHSFYHADCHLEPVRCQAPPPRSPSEASGRTVGSGKVYPTVHTSPPPEMLKDKALVEVAPSPGPPTLTSFNIPPGPFSSMHKLLETQSTGACHSSCKISSPCSKADSGACGPDSCPYCARTGAGEPESADHEMPDSDSEAVYEFTQDAQHSDLRDPHRRRRPSLGPDAEPSSVLAFWRLICDTFRKIVDSKYFGRGIMIAILVNTLSMGIEYHEQPEELTNALEISNIVFTSLFALEMLLKLLVYGPFGYIKNPYNIFDGVIVVISVWEIVGQQGGGLSVLRTFRLMRVLKLVRFLPALQRQLVVLMKTMDNVATFCMLLMLFIFIFSILGMHLFGCKFASERDGDTLPDRKNFDSLLWAIVTVFQILTQEDWNKVLYNGMASTSSWAALYFIALMTFGNYVLFNLLVAILVEGFQAEGDATKSESEPDFFSPSVDGDGDRKKRLALVALGEHSELRKSLLPPLIIHTAATPMSLPKSSSTGVGEALGSGSRRTSSSGSAEPGTAHHEMKSPPSARSSPHSPWSAASSWTSRRSSRNSLGRAPSLKRRSPSGERRSLLSGEGQESQDEEESSEEDRASPAGSDHRHRGSLEREAKSSFDLPDTLQVPGLHRTASGRSSASEHQDCNGKSASGRLARTLRADDPPLDGDDGDDEGNLSKGERLRAWVRARLPACCRERDSWSAYIFPPQSRFRLLCHRIITHKMFDHVVLVIIFLNCITIAMERPKIDPHSAERIFLTLSNYIFTAVFLAEMTVKVVALGWCFGEQAYLRSSWNVLDGLLVLISVIDILVSMVSDSGTKILGMLRVLRLLRTLRPLRVISRAQGLKLVVETLMSSLKPIGNIVVICCAFFIIFGILGVQLFKGKFFVCQGEDTRNITNKSDCAEASYRWVRHKYNFDNLGQALMSLFVLASKDGWVDIMYDGLDAVGVDQQPIMNHNPWMLLYFISFLLIVAFFVLNMFVGVVVENFHKCRQHQEEEEARRREEKRLKRLEKKRRNLMLDDVIASGSSASAASEAQCKPYYSDYSRFRLLVHHLCTSHYLDLFITGVIGLNVVTMAMEHYQQPQILDEALKICNYIFTVIFVLESVFKLVAFGFRRFFQDRWNQLDLAIVLLSIMGITLEEIEVNASLPINPTIIRIMRVLRIARVLKLLKMAVGMRALLDTVMQALPQVGNLGLLFMLLFFIFAALGVELFGDLECDETHPCEGLGRHATFRNFGMAFLTLFRVSTGDNWNGIMKDTLRDCDQESTCYNTVISPIYFVSFVLTAQFVLVNVVIAVLMKHLEESNKEAKEEAELEAELELEMKTLSPQPHSPLGSPFLWPGVEGVNSPDSPKPGAPHTTAHIGAASSGFSLEHPTMVPHTEEGPVPLGPDLLTVRKSGVSRTHSLPNDSYMCRNGSTAERSLGHRGWGLPKAQSGSILSVHSQPADTSCILQLPKDAHYLLQPHGAPTWGAIPKLPPPGRSPLAQRPLRRQAAIRTDSLDVQGLGSREDLLSEVSGPSCPLTRSSSFWGGSSIQVQQRSGSQSKVSKHIRLPAPCPGLEPSWAKDPQETRSSLELDTELSWISGDLLPSSQEEPLSPRDLKKCYSVEAQSCRRRPGSWLDEQRRHSIAVSCLDSGSQPRLCPSPSSLGGQPLGGPGSRPKKKLSPPSISIDPPESQGPRPPCSPGVCLRRRAPASDSKDPSASSPLDSTAASPSPKKDALSLSGLSSDPTDLDP
Inhibitor
Name:
BDBM50514579
Synonyms:
CHEMBL4575063
Type:
Small organic molecule
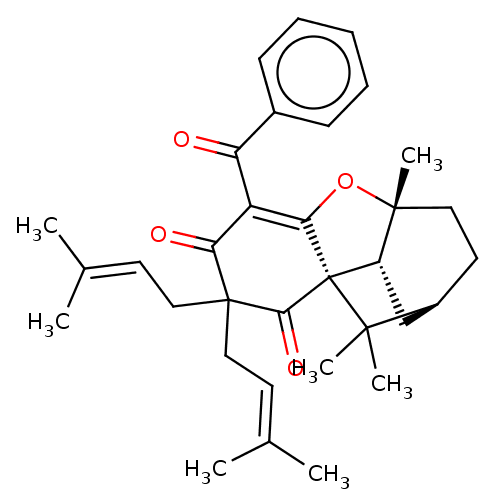
Emp. Form.:
C33H40O4
Mol. Mass.:
500.6683
SMILES:
[H][C@@]12[#6][C@@]3([H])[C@@]([#6])([#6]-[#6]1)[#8]-[#6]1=[#6](-[#6](=O)-c4ccccc4)-[#6](=O)C([#6]\[#6]=[#6](\[#6])-[#6])([#6]\[#6]=[#6](\[#6])-[#6])[#6](=O)[C@]31C2([#6])[#6] |r,c:11|