Reaction Details Report a problem with these data
Report a problem with these data
 Report a problem with these data
Report a problem with these dataTarget
Tyrosine-protein kinase BTK
Ligand
BDBM291455
Substrate
n/a
Meas. Tech.
ChEMBL_1873903 (CHEMBL4375192)
IC50
9.9±n/a nM
Citation
 Caldwell, RD; Qiu, H; Askew, BC; Bender, AT; Brugger, N; Camps, M; Dhanabal, M; Dutt, V; Eichhorn, T; Gardberg, AS; Goutopoulos, A; Grenningloh, R; Head, J; Healey, B; Hodous, BL; Huck, BR; Johnson, TL; Jones, C; Jones, RC; Mochalkin, I; Morandi, F; Nguyen, N; Meyring, M; Potnick, JR; Santos, DC; Schmidt, R; Sherer, B; Shutes, A; Urbahns, K; Follis, AV; Wegener, AA; Zimmerli, SC; Liu-Bujalski, L Discovery of Evobrutinib: An Oral, Potent, and Highly Selective, Covalent Bruton's Tyrosine Kinase (BTK) Inhibitor for the Treatment of Immunological Diseases. J Med Chem 62:7643-7655 (2019) [PubMed] Article
Caldwell, RD; Qiu, H; Askew, BC; Bender, AT; Brugger, N; Camps, M; Dhanabal, M; Dutt, V; Eichhorn, T; Gardberg, AS; Goutopoulos, A; Grenningloh, R; Head, J; Healey, B; Hodous, BL; Huck, BR; Johnson, TL; Jones, C; Jones, RC; Mochalkin, I; Morandi, F; Nguyen, N; Meyring, M; Potnick, JR; Santos, DC; Schmidt, R; Sherer, B; Shutes, A; Urbahns, K; Follis, AV; Wegener, AA; Zimmerli, SC; Liu-Bujalski, L Discovery of Evobrutinib: An Oral, Potent, and Highly Selective, Covalent Bruton's Tyrosine Kinase (BTK) Inhibitor for the Treatment of Immunological Diseases. J Med Chem 62:7643-7655 (2019) [PubMed] Article More Info.:
Target
Name:
Tyrosine-protein kinase BTK
Synonyms:
AGMX1 | ATK | Agammaglobulinaemia tyrosine kinase | Agammaglobulinemia tyrosine kinase | B cell progenitor kinase | B-cell progenitor kinase | BPK | BTK | BTK_HUMAN | Bruton tyrosine kinase | Tyrosine Kinase BTK | Tyrosine-protein kinase (BTK) | Tyrosine-protein kinase BTK (BTK)
Type:
Enzyme
Mol. Mass.:
76289.95
Organism:
Homo sapiens (Human)
Description:
Q06187
Residue:
659
Sequence:
MAAVILESIFLKRSQQKKKTSPLNFKKRLFLLTVHKLSYYEYDFERGRRGSKKGSIDVEKITCVETVVPEKNPPPERQIPRRGEESSEMEQISIIERFPYPFQVVYDEGPLYVFSPTEELRKRWIHQLKNVIRYNSDLVQKYHPCFWIDGQYLCCSQTAKNAMGCQILENRNGSLKPGSSHRKTKKPLPPTPEEDQILKKPLPPEPAAAPVSTSELKKVVALYDYMPMNANDLQLRKGDEYFILEESNLPWWRARDKNGQEGYIPSNYVTEAEDSIEMYEWYSKHMTRSQAEQLLKQEGKEGGFIVRDSSKAGKYTVSVFAKSTGDPQGVIRHYVVCSTPQSQYYLAEKHLFSTIPELINYHQHNSAGLISRLKYPVSQQNKNAPSTAGLGYGSWEIDPKDLTFLKELGTGQFGVVKYGKWRGQYDVAIKMIKEGSMSEDEFIEEAKVMMNLSHEKLVQLYGVCTKQRPIFIITEYMANGCLLNYLREMRHRFQTQQLLEMCKDVCEAMEYLESKQFLHRDLAARNCLVNDQGVVKVSDFGLSRYVLDDEYTSSVGSKFPVRWSPPEVLMYSKFSSKSDIWAFGVLMWEIYSLGKMPYERFTNSETAEHIAQGLRLYRPHLASEKVYTIMYSCWHEKADERPTFKILLSNILDVMDEES
Inhibitor
Name:
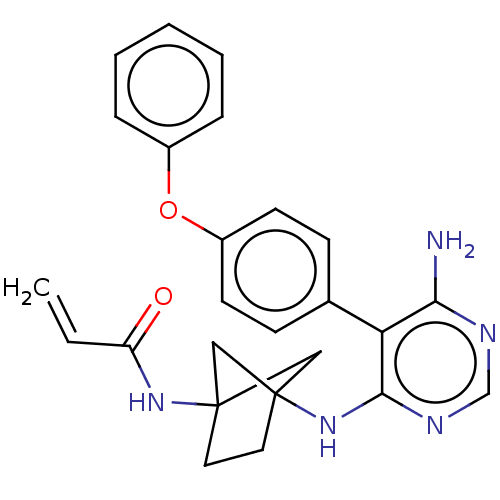
BDBM291455
Synonyms:
N-(4-((6-amino-5-(4-phenoxyphenyl)pyrimidin-4-yl)amino)bicyclo[2.1.1]hexan-1-yl)acrylamide | US10413562, Compound A177 | US9580449, Example A177
Type:
Small organic molecule
Emp. Form.:
C25H25N5O2
Mol. Mass.:
427.4983
SMILES:
Nc1ncnc(NC23CC(C2)(CC3)NC(=O)C=C)c1-c1ccc(Oc2ccccc2)cc1 |(-.82,-6.28,;-2.15,-5.51,;-3.48,-6.28,;-4.82,-5.51,;-4.82,-3.97,;-3.48,-3.2,;-3.48,-1.66,;-4.82,-.89,;-4.82,.65,;-6.28,1.13,;-5.88,-.36,;-7.19,-.12,;-6.28,-1.37,;-6.68,2.61,;-5.59,3.7,;-4.1,3.3,;-5.99,5.19,;-4.9,6.28,;-2.15,-3.97,;-.82,-3.2,;.52,-3.97,;1.85,-3.2,;1.85,-1.66,;3.19,-.89,;4.52,-1.66,;4.52,-3.2,;5.85,-3.97,;7.19,-3.2,;7.19,-1.66,;5.85,-.89,;.52,-.89,;-.82,-1.66,)|